ArtÃculo de Revisión
Intersocietal Argentine Pelvic Congestion Syndrome Consensus. Part 2
Miguel Amore, Hernán Bertoni, Pamela Causa Andrieu, Luis Catalina, Carolina Chacon, Carlos D´Alotto, Marcelo Dándolo, Guillermo Eisele, Santiago Gil, Néstor Giráldez, Sebastián Gogorza, Oscar Gural, Alberto Kenny, Esteban Mendaro, Noelia Napoli, Juan Nigro, Juan Paolini, Eugenio Piraino
Revista Argentina de Cardioangiología Intervencionista 2021;(1): 0014-0043 | Doi: 10.30567/RACI/20211/0014-0043
Este artículo no contiene abstract
Los autores declaran no poseer conflictos de intereses.
Fuente de información Colegio Argentino de Cardioangiólogos Intervencionistas. Para solicitudes de reimpresión a Revista Argentina de CardioangiologÃa intervencionista hacer click aquí.
Recibido | Aceptado | Publicado

Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-SinDerivar 4.0 Internacional.

















AUTHORS:
• Miguel Amore. miguelangelamore@hotmail.com.
2do Jefe de Servicio de Flebología y Linfología, Dto. de Cirugía Cardiovascular. Hospital Militar Central.
Staff del Servicio de Flebología y Linfología. Fundación Favaloro.
• Hernán Bertoni. hernangbertoni11@gmail.com.
Radiólogo Intervencionista. Jefe de Servicio Oncointervencionismo. Instituto Roffo.
Médico de Staff del Servicio de Cardioangiología Intervencionista. Instituto Fleni.
• Pamela Causa Andrieu. pamela.causa@hospitalitaliano.org.ar.
Médica Asociada. Servicio de Diagnóstico por Imágenes. Hospital Italiano de Buenos Aires, Argentina.
Clinical Fellow. Department of Radiology. Memorial Sloan Kettering Cancer Center, Nueva York, EE.UU.
• Luis Catalina. luismc12@gmail.com.
Médico especialista en Diagnóstico por Imágenes. Universidad de Buenos Aires.
Staff del Servicio de Ecografía Vascular. Fundación Favaloro y Diagnóstico Maipú.
Director Médico. Centro Diagnóstico Doppler San Miguel.
Encargado de la Sección Ecodoppler Abdominal y Pélvico. Vascular Integral.
Docente de SAUMB.
Miembro del Grupo Iberoamericano de Estudio Pélvico
• Carolina Chacon. carolina.chacon@hospitalitaliano.org.ar.
Médica de planta. Jefa de Sección de Ecografía. Coordinadora del Área de Imágenes en Ginecología. Servicio de Diagnóstico por Imágenes. Hospital Italiano de Buenos Aires, Argentina.
• Carlos D´Alotto. carlosdalotto@gmail.com.
Médico Especialista en Diagnóstico por Imágenes. Coordinador Área de Doppler vascular. Diagnóstico Maipú. Buenos Aires, Argentina.
• Marcelo Dándolo. mdandolo@gmail.com.
Cirujano Vascular. Expresidente de la Asociación Argentina de Angiología y Cirugía Cardiovascular (AAAyCCV).
Expresidente del Colegio Argentino de Cirugía Venosa y Linfática (CACVyL).
Subjefe del Servicio de Flebología y Linfología. Fundación Favaloro.
Staff de la Unidad de Cirugía Vascular. Hospital Perón y Sanatorio Itoiz.
Miembro Titular del Colegio Argentino de Cirujanos Cardiovasculares (CACCV).
Miembro Titular de la Asociación Argentina de Cirugía.
Miembro Titular del Grupo Iberoamericano de Estudio Pélvico.
• Guillermo Eisele. guillermoeisele@gmail.com.
Médico Especialista en Diagnóstico por Imágenes UBA y homologación España.
Jefe de Radiología Intervencionista. Hospital de Niños Dr. Ricardo Gutiérrez.
Miembro fundador Colegio Argentino de Radiología Vascular e Intervencionista.(CARVI).
Docente del Curso Superior de Diagnóstico por Imágenes. Hospital de Clínicas, UBA.
• Santiago Gil. santiago.gil@hospitalitaliano.org.ar.
Médico ginecólogo especialista en fertilidad y jefe de sección de Patología Pelviana Benigna. Hospital Italiano de Buenos Aires
• Néstor Giráldez. nestorgiraldez@yahoo.com.ar.
Cirujano Vascular. Miembro titular del Colegio Argentino de Cirujanos Cardiovasculares de la Sociedad de Flebología y Linfología Bonaerense y de la Asociación Argentina de Angiología y Cirugía Vascular.
Docente de la Carrera Universitaria de Flebología y Linfología. Universidad de Morón.
Unidad de Cirugía Vascular. Sanatorio Municipal Dr. Julio Méndez.
• Sebastián Gogorza. sebastian.gogorza@hospitalitaliano.org.ar.
Doctor en Medicina. Jefe honorario de Ginecología. Hospital Italiano.
Profesor Titular de Ginecología. Instituto Universitario Hospital Italiano de Buenos Aires.
• Oscar Gural. oagural@gmail.com.
Especialista en Cirugía Cardiovascular. Intervencionismo Venoso. Jefe de Servicio de Flebolinfología. Flebología Intervencionista. Fundación Favaloro.
Vicepresidente del Grupo Iberoamericano de Estudio Pélvico.
• Alberto Kenny. alberto.kenny85@gmail.com.
Médico Especialista en Diagnóstico por Imágenes y Radiología Intervencionista. Hospital Italiano, UBA. Becario Hospital Georges Pompidou, París, Francia.
Staff de Radiología Intervencionista de Sanatorios de Galeno Argentina y Swiss Medical Group, Buenos Aires, Argentina.
• Esteban Mendaro. esteban.mendaro@hospitalitaliano.org.ar.
Radiólogo Intervencionista. Director Médico de Investigaciones Vasculares.
Jefe del Servicio de Hemodinamia. Sanatorio de la Providencia.
Médico asociado de Diagnóstico por Imágenes. Hospital Italiano de Buenos Aires.
• Noelia Napoli. maria.napoli@hospitalitaliano.org.ar.
Médica Asociada. Servicio de Diagnóstico por Imágenes. Hospital Italiano de Buenos Aires, Argentina.
• Juan Nigro. juananigro@hotmail.com.
Cirujano Vascular Periférico. Director del Curso Superior Universitario de Flebología y Linfología, Universidad de Morón.
Director del Curso Superior de Ecodoppler Vascular Periférico e Intervencionismos Ecodirigidos (CACCV).
Vicepresidente de la Asociación Argentina de Angiología y Cirugía Cardiovascular (AAAyCCV).
Jefe de Unidad de Flebología y Linfología, Hospital Eva Perón, provincia de Buenos Aires.
Secretario general del CACCV
• Juan Paolini. juanestebanpaolini@gmail.com.
Cirujano Vascular Sanatorio Dr. Julio Méndez, Policlínico del Docente.
Presidente Colegio Argentino de Cirujanos Cardiovasculares (CACCV).
Presidente Argentine Chapter of Society of Vascular Surgery (SVS).
Secretario General Asociación Latinoamericana de Cirugía Vascular (ALCVA).
Secretario General Sociedad de Flebología y Linfología Bonaerense (SFLB).
Subdirector Curso Universitario de Flebología y Linfología, Universidad de Morón (UM)
• Eugenio Piraino. eepiraino@hotmail.com.
Médico ginecólogo especialista en Laparoscopia e Histeroscopia, Jefe de Servicio de Ginecología del Sanatorio San José.
• Damián Simonelli. dasimonelli@yahoo.com.ar.
Médico Cirujano General e Intervencionista, Servicio de Hemodinamia y Cirugía de CEMIC.
Staff de Radiología Intervencionista. sanatorios de Galeno Argentina y Swiss Medical Group, Sanatorio Mater Dei, Sagrado Corazón, Otamendi, Clínica San Camilo, Buenos Aires, Argentina.
• Thiago Vasconcellos. tvasconcelos@cdrossi.com.ar.
Médico, especialista en Diagnóstico por Imágenes. Centro Rossi, Buenos Aires, Argentina.
REVIEWERS
• Eduardo Eyheremendy
• Alejandro Kornberg
• Javier Leal Monedero
• Sergio Sierre
• Ernesto Torresani
• Santiago Zubicoa Ezpeleta
CACI COORDINATORS
• Arturo Fernández Murga
• Juan Manuel Ponce
GENERAL COORDINATOR
• Guillermo Eisele
PARTICIPANT MEDICAL SOCIETIES
• AAAyCCV: Argentine Association of Angiology and Vascular
Surgery.
• CACI: Argentine College of Interventional Cardioangiologist.
• CACCV: Argentine College of Cardiovacular Surgeons.
• CACVyL: Argentinian College of Venous and Lymphatic Surgery.
• CARVI: Argentine College of Vascular and Interventional Radiology.
• SAR: Argentine Society of Radiology.
• SFLB: Bonaerense Society of Phebology and Lymphology.
• Ibero-American Working Group of Pelvic Studies.
TABLE OF CONTENTS
1. Methodology used in the consensus document
1.1 Introduction
1.2 Methodology
2. Abbreviations
3. Summary of Consensus
3.1 Overview
3.2 Pathophysiology
3.3 Clinical signs
3.4 Differential diagnosis
3.5 Imaging diagnosis
3.6 Endovascular treatment
3.7 Medical treatment
3.8 Surgical treatment
3.9 Additional extrapelvic management
4. Intersocietal Argentine Pelvic Congestion
Syndrome Consensus
4.1 History and epidemiology
4.1.1 History
4.1.2 Epidemiology
4.1.3 References
4.2 Pathophysiology and clinical signs
4.2.1 Pathophysiological mechanisms
4.2.1.1 Introduction
4.2.1.2 Pathophysiology
4.2.2 Clinical signs
4.2.2.1 Differential diagnosis
4.2.2.2 Clinical evaluation of the results of the
therapies used
4.2.2.3 AVLS Classification
4.2.3 Recommendations
4.2.4 References
4.3 Diagnostic imaging modalities
4.3.1 Color-coded Doppler echocardiography
4.3.1.1 Introduction
4.3.1.2 Study protocol
4.3.1.3 Access routes and patient preparation
4.3.1.4 Diagnostic criteria for vascular compression
syndromes
• The nutcracker syndrome
• The May-Thurner syndrome
4.3.1.5 Diagnostic criteria for pelvic varicocele
• Gonadal veins
• Hypogastric or internal iliac veins
4.3.1.6 Pelvic leak points
4.3.1.7 Color-coded Doppler echocardiography
during postoperative management 

4.3.1.8 Conclusion
4.3.1.9 Recommendations
4.3.1.10 References
4.3.2 Computed axial tomography and magnetic
resonance imaging
4.3.2.1 Indications
4.3.2.2 Technique
4.3.2.3 How to request the study
4.3.2.4 Results
4.3.2.5 Summary
4.3.2.6 Recommendations
4.3.2.7 References
4.3.3 Gonadal and iliac dynamic phlebography
4.3.3.1 Introduction
4.3.3.2 Anatomy
4.3.3.3 Gonadal and iliac dynamic phlebography
• Generalities
• Technique
• Results
4.3.3.4 Recommendations
4.3.3.5 References
4.4 Importance of medical treatment
4.4.1 Introduction
4.4.2 The pelvic congestion syndrome as the cause of chronic pelvic pain
4.4.3 Medical therapy
4.4.3.1 Non-steroidal anti-inflammatory drugs
4.4.3.2 Ergotamine
4.4.3.3 Hormonal therapy
4.4.3.4 Venoactive drugs
4.4.3.5 Compression treatment for pelvis and lower
limbs.
4.4.4 Recommendations
4.4.5 References
4.5 Endovascular treatment
4.5.1 Introduction
4.5.2 Transcatheter embolization
4.5.2.1 Principles
4.5.2.2 Technique and materials
4.5.2.3 Postembolization care, complications, and
follow-up
4.5.2.4 Recommendations for transcatheter embolization of gonadal veins and hypogastric vein branches
4.5.2.5 Results
4.5.3 Angioplasty in venous obstruction syndromes
4.5.3.1 Obstruction of inferior vena cava and
May-Thurner syndrome
• Pathophysiology and clinical presentation
• Technique
• Results
4.5.3.2 The nutcracker syndrome
• Phenomenon, syndrome, and differential diagnosis
• Treatment
• Technique
• Results
4.5.4 Recommendations
4.5.5 References
4.6 Surgical treatment
4.6.1 Introduction
4.6.2 Conservative surgical treatment
4.6.2.1 Selective gonadal vein ligation through open or conventional approach
4.6.2.2 Laparoscopic selective gonadal vein ligation
• Complications of laparoscopic gonadal ligation
• Limitations of laparoscopic gonadal ligation
4.6.3 Non-conservative surgical treatment
4.6.3.1 Hysterectomy and salpingo-oophorectomy
4.6.3.2 Complications of open surgery
4.6.4 Vascular and endovascular surgical treatment of compression syndromes as the cause of the pelvic congestion syndrome
4.6.4.1 The May-Thurner syndrome
4.6.4.2 The nutcracker syndrome
• Pathophysiology and clinical signs
• Treatment options of the nutcracker syndrome
o Conservative treatment
o Interventional behavior
4.6.5 Analysis and considerations
4.6.6 Recommendations
4.6.7 References
4.7 Additional extrapelvic treatment
4.7.1 Introduction
4.7.2 Comprehensive diagnostic evaluation of pelvic venous incompetence
4.7.2.1 Clinical-semiological examination
4.7.2.2 Semiological examination under augmented reality
4.7.2.3 Color-coded Doppler echocardiography
4.7.2.4 Computed axial tomography and magnetic resonance imaging
4.7.2.5 Gonadal and iliac dynamic phlebography of pelvic floor leak
4.7.2.6 Varicography
4.7.3 Therapeutic arrangement of lower limb venous incompetence originated from pelvic varicose veins reflux
4.7.3.1 Common pelvic floor leak points
4.7.3.2 Treatment techniques of pelvic floor leak points
• Percutaneous extrapelvic embolization
• Section and selective ligation of leak points
associated with intra- and extrapelvic sclerosis
4.7.4 Postoperative management
4.7.5 Treatment of intrafascial and epifascial
vessels
4.7.5.1 Conventional surgery
4.7.5.2 Minimally invasive procedures
4.7.6 Analysis and considerations
4.7.7 Recommendations
4.7.8 References
4.4 Importance of medical treatment
4.4.1 Introduction
Chronic pelvic pain (CPP) is a relatively common situation that affects women of reproductive age, negatively impacts quality of life, and is associated with high healthcare costs. Although there is not such thing as a universally accepted definition, the one most commonly used is: “cyclical or non-cyclical pelvic pain of more than 3 to 6 months of evolution intense enough to cause functional disability and require treatment” (1-3).
The prevalence of CPP goes from 2% to 25% based on different definitions used and different populations studied; in any case, it is similar to the prevalence of migraine, lower back pain or asthma (4,5).
According to some reports, CPP represents up to 40% of all gynecological consultations, and it is one of the main causes for laparoscopy in the gynecology setting (8).
These figures may seem biased since they may refer to centers devoted to treat CPP or endometriosis, and although there are no statistics for Argentina on this regard, in our daily routine practice, these numbers seem much lower than they should.
In addition to pelvic pain, the patient with CPP can present with dysmenorrhea, dyspareunia, dysuria, dyschezia, lower back pain, and vulvar or perineal pain.
CPP can be due to multiple potential etiologies; as a matter of fact, in many cases, these etiologies overlap. It is also a fact that in a high percentage of patients (up to 50%) no organic cause is found as the origin of pain (6). Also, as it happens in other clinical manifestations of chronic pain, there is a high correlation with psychosocial factors (6). For all these reasons, the diagnostic approach and subsequent treatment should be conducted by a multidisciplinary team.
The potential organic etiologies of CPP can be endometriosis, irritable bowel syndrome, bladder pain syndrome, adenomyosis, adherences, nerve entrapment, myofascial syndrome, and PCS. As a matter of fact, the same patient can show more than one single cause.
PCS is often a misdiagnosed cause for CPP. According to current medical literature available, up to 10% of women of reproductive age have pelvic varicose veins, and a percentage of them (up to 60%) will end up developing PCS (9).
A prevalence of PCS from 10% to 30% has been reported in women with CPP without any other obvious cause of pain (9,11).
Women with PCS can show symptoms that are common to other causes of CPP. However, some of them are specific to this condition:
• Dull pain or feeling of predominantly unilateral pelvic heaviness.
• Pain is more intense at the end of the day but improves while resting in the supine position.
• Pain can exacerbate with menstruation, with situations that increase intra-abdominal pressure(such as, for example, weightlifting), with long periods standing up, with sexual intercourse or with successive pregnancies.
During physical examination, certain signs suggestive CPP have been reported, still they are not exclusive to this condition.10 Only the finding of vulvovaginal, perineal, gluteal or LL varicose veins is suggestive of this condition.
4.4.2 The pelvic congestion syndrome as the cause of chronic pelvic pain
The pathophysiology of PCS is not clear, and it is probably multifactorial. The incompetence of ovarian and/or internal iliac veins and their tributaries is the necessary condition. Valvular incompetence (whether congenital or acquired), venous obstruction (whether intrinsic or extrinsic), and hormones play a key role in the development of PCS (7,12).
Venous incompetence causes stasis, reflux, increases pressure, endothelial and venous wall damage, and eventually dilatation and venous impossibility to contract and relax.
The vessel wall endothelial and muscular cells have estrogen receptors. Estrogens produce vasodilatation through different mechanisms. It is unclear what their role is in the genesis of pelvic varicose veins, but this association was established in different situations, for example, in female patients who reach menopause or receive different therapies that result in hypoestrogenemia, the painful symptoms associated with PCS improve.
The correlation of venous dilatation as a cause of CPP is still under discussion (7,12).
Regardless of the cause of venous incompetence is, the clinical symptoms can be attributed to two different mechanisms:
• The dilatation of pelvic veins can activate pain receptors on the venous walls.
• The release of neurotransmitters of the dilated venous walls (substance P, a calcitonin gene-related peptide, adenosine triphosphate, endothelin, vasopressin, nitric oxide) involved in the responses to pain.
4.4.3 Medical therapy
As already mentioned, the cause of PCS is possibly multifactorial; and pelvic venous incompetence and dilatation, mechanical factors (venous obstruction), and the hormonal status play a significant role in the development of this syndrome.
Therefore, the logical treatment is the use of vasoconstrictors, hormonal status-modifying drugs by creating a hypoestrogenic environment or procedures that ligate or occlude the varicose veins.
To initiate the medical treatment of PCS first it is necessary to rule out other causes of CPP. A multidisciplinary team including gynecologists, urologists, gastroenterologists, radiologists, neurologists, and psychologists is required to rule out other causes of CPP.
4.4.3.1 Non-steroidal anti-inflammatory drugs (NSAID)
Analgesic drugs are often the first-line therapy (whether indicated or self-medicated) to alleviate painful manifestations in general. In cases of PCS, NSAID bring relief to some patients, but their long-term use is limited by their adverse events (GI and blood-related). They can be used while the patient is undergoing diagnostic tests, and only until a more permanent treatment is established (13,14).
4.4.3.2 Ergotamine
This drug is an ergot alkaloid (rye ergot fungus) that targets serotonin, dopamine, and adrenaline receptors causing, among other effects, vasoconstriction. It is currently used in the form of nasal sprays, subcutaneous, intramuscular and IV injections to treat migraine. Ergometrine is used to prevent and treat postpartum bleeding.
It has been reported that, in the management of PCS, it improves up to 30% of painful symptoms after its IV administration. Its short therapeutic effect and possible adverse events limit its long-term use (15,16).
The following contraindications are often described: known hypersensitivity to rye fungus alkaloids, pregnancy, breastfeeding, kidney and liver failure, peripheral and coronary artery disease, obliterative vascular diseases, Raynaud’s syndrome, temporal arteritis, severe or uncontrolled hypertension, hyperthyroidism, and porphyry.
4.4.3.3 Hormonal therapy
Progestogens
Different progestogens have been widely studied and used to treat of other causes of PCS, especially endometriosis.
Medroxyprogesterone acetate (MPA) has been used to treat PCS. It is a synthetic byproduct of progesterone that has antiestrogenic and antigonadotropic properties.
At certain doses, it inhibits ovulation causing amenorrhea. It has been used a contraceptive, to treat dysmenorrhea, endometriosis, in hormone replacement therapy, and for the management of end-stage endometrial, breast and kidney cancer. It is active via oral and parenteral routes of drug administration.
Several former studies have proven its effectiveness alleviating the symptoms associated with PCS and reducing objectively abnormal pelvic venogram scores (7,18). The subdermal implantation of etonogestrel (derived from desogestrel), a progesterone that blocks follicular development and ovulation, generates a hypoestrogenic state. It is used as a contraceptive, and thanks to its therapeutic action, it can benefit patients with PCS (19).
Combined oral contraceptives, progesterone pill only,
and levonorgestrel-releasing intrauterine system (LNG-IUS)
Combined oral contraceptives (estrogens and progesterone) and progesterone pills only create a hormonal environment that is mainly progestational with a relative decrease in the levels of estrogens, which can benefit patients with PCS. They have been studied apart from their contraceptive function, and they have proven effective to treat other gynecological conditions responsible for chronic pelvic pain like endometriosis and adenomyosis. However, they have not been studied to treat PCS related symptoms.
The LNG-IUS, developed initially as a contraceptive, has turned out to be very effective to treat abnormal uterine bleeding. Its indication has been expanded to treat other conditions like endometriosis, adenomyosis, and endometrial hyperplasia, where it also proved effective. Although it shares therapeutic effects with other progesterone drugs, it has not been studied in patients with PCS.
GnRH agonists
Gonadotropin releasing hormone (GnRH) is a decapeptide produced by hypothalamic neurons that is released in a pulsatile manner towards the portal circulation of hypophysis where it regulates the release of FSH and LH. These gonadotropins target the ovaries during follicular development and subsequent ovulation.
Numerous GnRH analogs have been synthesized (leuprolide, goserelin, decapeptyl, nafarelin, buserelin, etc.) introducing changes in positions 6 and 10 of the decapeptide chain. They are active via nasal, subcutaneous, and intramuscular routes of drug administration. The ongoing application of this medication blocks hypophyseal receptors and inhibits the production of FSH and LH, which prevents follicular development and ovulation causing a menopause-like hypoestrogenic state. They have been used to treat different benign and malignant gynecological conditions over the years. They have been used to treat PCS related painful symptoms, but their adverse events (climacteric symptoms and osteoporosis) limit their long-term use (17).
4.4.3.4 Venoactive drugs
Venoactive drugs (micronized purified flavonoid fraction of diosmin-hesperidin) increase venous tone and capillary resistance, improve lymphatic drainage, and reduce capillary patency, which reduces venous stasis. It is a safe and well-tolerated drug that is indicated alone or in combination with other therapies to alleviate the symptoms of LL venous incompetence.
Some studies have already confirmed the effectiveness of this drug reducing PCS related pelvic pain. Symptoms improve slowly but persistently on a monthly basis once the drug has been discontinued (20-22).
4.4.3.5 Compression treatment for pelvis and lower limbs
The use of compression pants and stockings has brought clinical improvement to over 80% of the cases of patients with PCS and LL varicose veins (23).
4.4.4 Recommendations
|
1 |
Non-steroidal anti-inflammatory drugs can be initially used to treat PCS related pain while the patient is waiting for more permanent therapies. (Level of Evidence B-C, Recommendation Class II a-b). |
|
2 |
Ergotamine can improve PCS related pain symptoms. Its short therapeutic effect and adverse events limit its use. (Level of Evidence B, Recommendation Class II a-b). |
|
3 |
Hormonal treatment with progesterone and GnRH analogues can improve PCS related pain but the adverse events limit the prolonged used of these drugs. (Level of Evidence B-C, Recommendation Class II a-b). |
|
4 |
Venoactive drugs (diosmin) improve PCS related pain. (Level of Evidence B, Recommendation Class II a-b). |
4.4.5 References
- Williams RE, Hartmann KE, Steege JF. Documenting the current definitions of chronic pelvic pain: implications for research. Obstet Gynecol 2004;103:686-91.
- Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv 1993;48:357-87.
- International Association for the Study of Pain Taxonomy Working Group. Classification of Chronic Pain. Seattle: IASP; 2014.
- Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity.BMC Public Health 2006,6:177.
- Ahangari A. Prevalence of Chronic Pelvic Pain Among Women: An Updated Review. Pain Physician 2014;17:141-7.
- Daniels J, Khan K. Chronic pelvic pain in women. BMJ 2010;341:772-5.
- Champaneria R, Shah L, Moss J, Gupta J, et al. The relationship between pelvic vein incompetence and chronic pelvic pain in women: systematic reviews of diagnosis and treatment effectiveness. Health Technol Assess 2016;20(5).
- Brown C, Rizer M, Alexander R, et al. Pelvic Congestion Syndrome: Systematic Review of Treatment Success. Semin Intervent Radiol 2018;35:35-40.
- Borghi C, Dell’Atti L. Pelvic congestion syndrome: the current state of the literature. Arch Gynecol Obstet 2016;293:291-301.
- Beard RW, Reginald PW, Wadsworth J. Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol 1988;95(2):153-61.
- Meissner MH, Gibson K. Clinical outcome after treatment of pelvic congestion syndrome: Sense and nonsense. Phlebology 2015;30(suppl 1):73-80.
- Phillips D, Deipolyi A, Hesketh R, et al. Pelvic Congestion Syndrome: Etiology of Pain, Diagnosis, and Clinical Management. J Vasc Interv Radiol 2014;25:725-33.
- Nicholson T, Basile A. Pelvic congestion syndrome, who should we treat and how? Tech Vasc Interv Radiol 2006;9(1):19-23.
- Taskin O, Sahin L; Gavrilov S, Lazarashvili Z. Medical treatment of pelvic congestion síndrome. Phlebolymphology 2016;23(3):146-53.
- Reginald P, Beard R, Kooner J, et al. Intravenous dihydroergotamine to relieve pelvic congestion with pain in young women. Lancet 1987;2:351-3.
- Taskin O, Sahin L; Gavrilov S, Lazarashvili Z. Medical treatment of pelvic congestion síndrome. Phlebolymphology 2016;23(3):146-53.
- Soysal ME, Soysal S, Vicdan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod 2001;16(05):931-9.
- Farquhar CM, Rogers V, Franks S, Pearce S, Wadsworth J, Beard RW. A randomized controlled trial of medroxyprogesterone acetate and psychotherapy for the treatment of pelvic congestion. Br J Obstet Gynaecol 1989;96(10):1153-62.
- Shokeir T, Amr M, Abdelshaheed M. The efficacy of Implanon for the treatment of chronic pelvic pain associated with pelvic congestion: 1-year randomized controlled pilot study. Arch Gynecol Obstet 2009 Sep;280(3):437-43.
- Lyseng-Williamson K, Perry C. Micronised purified flavonoid fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs 2003;63:71-100.
- Simsek M, Burak F, Taskin O. Effects of micronized purified flavonoid fraction (Daflon) on pelvic pain in women with laparoscopically diagnosed pelvic congestion syndrome: a randomized crossover trial. Clin Exp Obstet Gynecol 2007;34:96-8.
- Gavrilov S, Karalkin A, Moskalenko E, et al. Micronized purified flavonoid fraction in treatment of pelvic varicose veins [in Russian]. Angiol Sosud Khir 2012;18(1):71-5.
- Gavrilov SG, Karalkin AV, Turischeva OO. Compression treatment of pelvic congestion syndrome. Phlebology 2018;33:418–24.
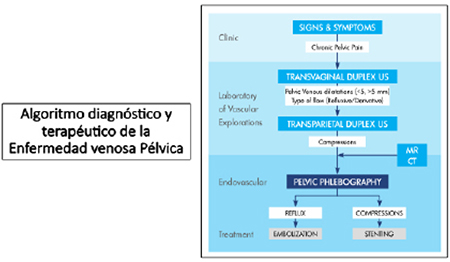
4.5 Endovascular treatment
Authors: Hernán Bertoni, Esteban Mendaro, Alberto Kenny
4.5.1 Introduction
The hypothesis that associates PCS pain and dilatation, venous stasis, and the release of local vasoactive substances has been confirmed by different studies (1). Therefore, the main therapeutic focus should be to correct the venous stasis of the incompetent dilated veins with different degrees of reflux.
Since the first description of the transcatheter embolization (TE) technique for the management of PCS back in 1993 (2), several reports and case series have presented TE as a safe, effective, minimally invasive therapeutic option with rates of technical success over 95%, and a 68% to 100% rate of symptom-relief (3,4). To this date, the diagnostic and therapeutic management of cases of PCS associated with pelvic venous reflux (PVR) is individualized, based on the needs of each patient while also taking into account symptom severity and the patterns of PVR (3-5).
PCS responds mainly to two pathophysiological mechanisms that can occur alone or together: 1) valvular dysfunction of gonadal and/or hypogastric veins often associated with leaks through collateral veins, and 2) (total or partial) pelvic vein obstruction (NCS, MTS, obstruction of IVC) (3) Depending on whether PCS is caused by one or the other, the endovascular treatment technique will change accordingly. TE should be the treatment of choice in cases of gonadal vein incompetence while PTCA with stenting should be the early treatment of choice in cases of obstructive phenomena (6,7).
Unlike conventional surgery, percutaneous endovascular procedures use a minimally invasive approach to treat incompetent veins. They are often performed in the cath lab on an outpatient basis under fluoroscopic guidance, local anesthesia or conscious sedation with the venous catherization technique. This facilitates the patient’s quick comeback to his routine activities and reduces significantly the high costs associated with this disease (8). The objective of this chapter is to expose the guidelines on endovascular treatments like TE and PTCA and assess the clinical results of patients with PCS.
4.5.2 Transcatheter embolization
4.5.2.1 Principles
The premise behind TE for the management of PCS due to PVR is that symptoms can alleviate by eliminating the venous reflow pathways that cause the unusually high hydrostatic pressure of uterine and ovarian veins and annexes. Therefore, the goal of TE is to occlude the main ovarian reflow and periuterine venous plexus veins, as well as the pelvic floor hypogastric branches that can cause PCS and LL leaks through the uterine vein.
Since these branches are numerous and difficult to see, the chances of relapse grow thinner after occluding the largest possible area of the incompetent diseased veins with reflow both in the utero-ovarian veins and the tributary branches of the internal iliac veins. It is important to occlude all those veins with confirmed reflow to avoid an incomplete clinical improvement. In the presence of pelvic floor leakage (PFL) into LL and/or the connections between the internal iliac veins andperiuterine varicose veins, a selective TE of these should be performed and the results monitored through time with a CDE performed by a skilled and trained operator (7-14).
4.5.2.2 Technique and materials
This procedure can be performed during the same day at the hospital on an outpatient basis, although some operators prefer to see the patients the day after the procedure to verify their clinical improvement. There is no evidence that the TE of the PCS should be scheduled to be performed at a given time during the menstrual cycle (9) or that routine antibiotic prophylaxis should be prescribed (10).
Local anesthesia at vascular access site level is often enough. However, there are times that mild conscious sedation can be useful to relax the patient, especially when the jugular access is used. Sedation should facilitate the voluntary control of the patient so that the Valsalva maneuver can be performed effectively during the administration of embolic-sclerosing agents.
Several access routes can be used to reach the abdominal and pelvic central veins via brachial and jugular accesses and secondly the femoral access. All of them have a high rate of technical success and a low rate of complications (3,7). After venous puncture preferably with an ultrasound-guided guidewire, a 5-Fr or 6-Fr introducer sheath is inserted using the Seldinger technique. These diameters facilitate an eventual coils retrieval in case of migration of malappositioning (10,11) as well as the use of plugs for large-caliber gonadal veins, when appropriate.
Both the ovarian and iliac vein selective catheterization can be performed using a 5-Fr preformed hydrophilic catheter (preferably a “multipurpose” catheter with a single terminal orifice for the brachial and jugular accesses) and a 0.035-in hydrophilic guidewire. There are times (more commonly when the femoral access is used) that a Cobra 2 or a Simmons 1 catheter is used for left (Cobra 2) and right (Simmons 1) gonadal vein catheterization, respectively; for a deep and distal hyperselective catheterization of ovarian veins, a microcatheter may be necessary. Actually, this can be determinant for the long-term technical and clinical success of the procedure (9-14). The coaxial microcatheter technique to treat small periuterine pelvic varicose veins and vulvar varicose veins can also be useful. Depending on the access used and/or venous anatomy, a long vascular introducer sheath or guide catheter can be used to stabilize the whole system (11).
Pelvic varicose vein occlusion can be achieved through the endothelial damage caused by the mechanical, “detergent-like” or osmotic action from different embolic and sclerosing agents. The mechanical devices for gonadal and hypogastric vein occlusion and their branches include coils (Figures 1, and 2), occluders or vascular plugs (Figure 3) with or without associated sclerotherapy and rarely cyanoacrylate adhesive embolization. Although we don’t have solid data on the superiority of one technique over the other (7), coils are the most widely used mechanical endovascular occlusion devices and are often used with sclerosing agents as the chemical method for varicose veins sclerotherapy (3-27). Coils work as a long-term surgical ligation in situ (11), that prevents the recanalization of vein. Sclerosing agents cause endothelial damage, endoluminal fibrosis, and, eventually, the occlusion of the incompetent varicose veins. The so-called sandwich technique when performing TE is often used in our setting as described by Dr. Leal Monedero and Dr. Zubicoa. It combines the use of aethoxysklerol or polidocanol foam and fiber coils starting at the most distal area with the use of foam. Coil delivery is followed by a second injection of proximal sclerosing foam. In the ovarian vein most proximal sector to be occluded, it is advisable to embolize up to 5 cm prior to entering the left renal vein or the inferior vena cava. This technique allows us to treat small affluent branches often associated with ovarian veins and the possible cause of recurrence (7,10).
The use of sclerosing agents reduces the number of coils needed to achieve an effective TE, which reduces the cost of therapy. Sclerosing agents can be injected as a liquid or foam (the bubbles in the foam carry the sclerosing agents). When injected as a foam, the sclerosing agent displaces blood facilitating a direct and more prolonged and extensive contact with the endothelium. However, when injected as a liquid the sclerosing agent dilutes with blood reducing the concentration reached in the vessel wall (17).
The most widely used sclerosing agents are aetoxisclerol or polidocanol in concentrations of 1% to 3%. The sclerosing foam can be prepared using two syringes and a three-way valve. Afterwards, a 1:1 to 1:4 liquid-to-air ratio is used with or without acontrast agent (the so-called Tessari method). When the sclerosing agent is not associated with a contrast agent, the target vein can initially be opacified and under fluoroscopy guidance the column of “positive” contrast can be displaced with the foam generating a column of “negative” contrast (17).
The coils most commonly used are spiral-shaped and made out of stainless steel or platinum. They are often pushable fiber coils. They are 0.018-in or 0.035-in caliber, 5 mm to 20 mm in diameter, and 7 cm to 40 cm in length. The controlled delivery of long coils of up to 40 cm in length for gonadal vein embolization is advisable especially the proximal portion, where precise positioning is required to avoid any protrusions inside unwanted vascular structures (LRV and IVC during the proximal TE of gonadal veins). Venous TE with coils to treat PCS requires to oversize the coils by 30%to match the diameter of the vein to be embolized in order to minimize the risk of migration. However, there is no need to compact the coils as it is the case with arterial aneurysms, which reduces significantly the number of coils needed for an effective TE; between 4 and 6 14cm long coils and 2 to 3 30 cm-to-40 cm long fiber coils are often needed to treat an ovarian vein (3-27) (Figures 4, 5, and 6).
Picture 4F shows the CDE mapping, the internal pudendal and left obturator veins PFL into the superficial varicose veins of the thigh antero-lateral side and leg posterior side. Picture 4G; the phlebography performed prior to treatment confirms leaks from incompetent internal pudendal (black arrows) and (Picture 4H) left obturator veins (white arrows). Picture 4I; post-TE using the sandwich technique with sclerosing agents and coils (C), and plug (P) The image confirms the closure of the leaks with prolonged stagnation of contrast during the Valsalva maneuver.
At times, plugs become useful tools to achieve effective and precise TEs in large-diameter blood vessels like in the gonadal and hypogastric veins proximal sectors. A randomized clinical trial recently compared the results of TE to treat PCS with fiber coils and plugs in 100 patients (21). Ovarian and hypogastric veins were treated with a 1-year rate of clinical success of 90% in both groups. Fewer devices were needed in the group treated with plugs (4 vs. 18), shorter fluoroscopy times, and less dose of radiation.
The combined TE of the gonadal vein can be performed through the injection of a sclerosing agent into the vein most distal portion followed by the delivery of coils or plugs as agents of the mechanical TE into the proximal portion. However, as already mentioned before, the mixed TE “sandwich” technique is the most commonly used and recommended one. For this purpose, ovarian vein catheterization should be deep and stable. The injection of the sclerosing agent should be stopped in the presence of mild reflow across the catheter distal portion; the simultaneous use of the Valsalva maneuver during the injection of foam prevents reflow into the proximal portion and improves the penetration and distality of collateral branches. Coil deliveryshould begin as distal as possible after the injection of the sclerosing agent. After treating the reflow most distal area, the catheter is gradually retrieved at a rate of 5 cm at a time, and coils are delivered in every level up to a few centimeters from the left renal vein and right IVC. The procedure is completed after the administration of a few more doses of foam, which creates some sort of “sandwich” between the coils and the sclerosing agent throughout the entire ovarian veins (7,18).
TE should extend to both gonadal veins entirely including these veins main tributary drainage branches to avoid recanalization though parietal collateral veins (Figure 7).
To this point, special attention should be paid to make sure that the sclerosing agent and the coils don’t migrate towards the LRV, IVC or pulmonary arteries; the Valsalva maneuver and the reverse Trendelenburg position during the injection of the sclerosing agent can minimize this risk. If possible, embolization should be bilateral using the same protocol in both ovarian veins (6-18). In cases of not so evident reflow in the right ovarian vein some authors propose performing the TE with coils or plugs. Other authors, however, suggest using foam in both gonadal veins distal sector, at all times, since they are intercommunicated (10,21).
Hypogastric veins should rather be catheterized via supradiaphragmatic access using a multipurpose catheter. Initially, a diagnostic phlebography of the hypogastric vein should be performed to confirm the presence of pelvic varicose veins and rule out the presence of leaks or reflows into the genital region or LL through the pudendal, gluteal, and obturator veins. After confirming the presence of leaks as seen on the phlebography, these incompetent branches should be selectively catheterized as distal as possible to perform the TE with micro-foam perfusion and coil delivery. Polidocanol foam is useful to treat smaller peripheral vulvar veins (11).
All the necessary precautions should be taken regarding the TE of distal branches of the hypogastric vein, mainly the superior and inferior gluteal veins, because of the large diameter they can reach, proximity to the main branches of the hypogastric vein, and potential risk of migration (Figure 8). To avoid this complication, it is necessary to use controlled delivery coils and place them as distal as possible. Also, they should be landed effectively in, at least, two venous branches using the sandwich technique, and target vessel oversizing (from 30% to 40%).
Some studies suggest using occlusion balloon catheters for the early diagnosis and treatment of hypogastric veins and their leaks into the LL and genital region. In these cases, the balloon catheter should remain inflated for, at least, 5 min. after the administration of the sclerosing agent in order to prevent reflow (9-14).
4.5.2.3 Postembolization care, complications, and follow-up
In some cases, after the TE treatment, pain can be quite debilitating. That’s why some authors prefer to hospitalize their patients for pain management and control assessments until the next day following the procedure (9-14). Patients can experience mild-to-moderate discomfort in the pelvic region for up to 5 days after the procedure (it is often temporary and rarely extends in time) with a good and quick response to NSAID (11). These symptoms are considered inherent to venous occlusion and known as “post-embolization syndrome” whose main symptoms are pain and low-grade fever. Both symptoms would be associated with the number and caliber of the vessels treated (6). Demanding physical activities should be avoided for 7 to 10 days after treatment (10).
TE complications associated with PCS are highly rare. The rate of complications is low (< 3%) and they are often minor complications including access vein thrombophlebitis, ovarian vein perforation, puncture site hematomas, a 13% rate of varicose recurrence at the 5-year follow-up, and coil migration. The latter is the most feared complication; it often resolves using endovascular techniques and the Amplatzer snare (Figure 9). The use of controlled delivery fiber coils has reduced their frequency considerably (4,6,16,28).
Although the authors have different views about it, both the control assessment and follow-up of patients post-TE have received very suitable proposals by the American Society of Interventional Radiology (16). The clinical results of TE can be assessed using visual analog scales (VAS) to measure the reduction of pelvic heaviness and pain. Also, quality of life (QOL) surveys can be used before and after treatment (6,16). It is advisable to make a consultation appointment 3 months after the procedure followed by a second visit 6 months later with the results of a transabdominal and transvaginal CDE and, if possible, with the results of a control MRI too (6,10).
4.5.2.4 Recommendations for transcatheter embolization of gonadal veins and hypogastric vein branches
• Choose the brachial or jugular venous access over the femoral one (better control, and lower risk or migration).
• Use femoral access in the retroaortic renal vein and when performing difficult right gonadal vein catheterizations.
• Use the “sandwich” technique (foam-coils-foam) during the TE.
• Perform distal-to-proximal TE.
• TE should be performed as proximal as possible to the gonadal veins to prevent relapses, and up to 5 cm away from the venous entrance while treating the entire incompetent venous axis and its bypass pathways.
• Use long controlled delivery coils with diameters that should exceed the target vessel significantly (oversize coils between 30% to 40%).
• Coils should be properly landed in large-caliber vessels with angulated controlled delivery coils that should also cover the two venous branches (Figure 10).
4.5.2.5 Results
Currently, TE is the method of choice to treat PCS in the absence of obstructive causes (7); the combination therapy of sclerotherapy and TE is a safe and effective method with a technical success rate >99%. In more that 90% of the cases, symptoms improve as confirmed by the VAS between pre- and post-treatment, and from 5 to 6 points on a scale from 0 to 10.4 (28). However, and although numerous case series and randomized clinical trials suggest that women with CPP due to ovarian or hypogastric vein reflux benefit from TE, overall, the evidence available to this point is of medium quality (3-27). The Latin American Therapeutic Guidelines on the management of Venous Disease and the American Venous Forum recommend endovascular treatment for PCS with an Intermediate Level of Evidence B and a Class I Recommendation (High Grade), being the first-line therapy particularly in young women (6,29).
Most authors agree on the importance of eliminating all sources of abdominal reflux into pelvic floor veins to minimize the risk of insufficient treatment and symptom recurrence (5-14). In cases of communication among the ovarian venous plexus, the internal iliac vein tributary veins, and PFL points into the LL, the TE of such venous connections improves the rate of success and reduces the rate of recurrence (14). No comparative studies have been conducted to this date so different views on this issue have come up: on the one hand, perform a more conservative TE limited to the gonadal veins; and on the other hand, perform a more aggressive treatment including the incompetent hypogastric venous branches of connection between pelvic floor and the LL (4). Some authors claim that the therapeutic failure of certain patients may be due to the avoidance of incompetent pelvic veins with PFL in the TE (5). If possible, refluxes should always be treated as proximal as possible.
The causes of relapse or recanalization of embolized veins are:
• Pregnancy.
• Weight gain.
• Technical failures:
- Inadequate, small coils that do not occlude the entire venous axis and its bypass pathways.
- Very proximal TE without distal treatment or vice versa. The delivery of very distal coils leaving a very long proximal venous segment untreated.
• Untreated associated compression syndromes such as the NCS or the MTS responsible for the secondary PCS.
Although there should be no significant differences in the clinical results of endovascular treatment compared to the surgical treatment of PCS, the minimally invasive nature of the procedure plays a key role when choosing the TE with short recovery times and very low rates of complications (6). Also, some authors suggest that the clinical results of endovascular treatment may be superior to the surgical ones in the management PCS (15,23), because it is very complicated to use the surgical approach on an incompetent gluteal vein with sciatic varicose veins.
4.5.3 Angioplastia en los síndromes obstructivos venosos
La obstrucción del drenaje venoso de la pelvis debe ser considerada y evaluada cuidadosamente en los casos de SCP. Las causas obstructivas-compresivas han sido subestimadas como etiología del SCP30 y tanto los fenómenos compresivos altos tipo SNC, como los bajos, tipo SMT y obstrucción de VCI, pueden provocar IVP, várices útero-ováricas y SCP6,7,30-37. La presencia de IVP y SCP en los casos de SMT tiene una incidencia variable de hasta 80%31, y frecuencia de 83% en el SNC32.
Ante la sospecha clínica y por imágenes (EDC, TAC o RM) de SCP asociado a SNC o SMT, la FDGI es el método de elección para confirmar el diagnóstico y que permite corroborar la existencia de gradiente de presión transestenótico de significancia (consultar los capítulos previos de diagnóstico por imágenes)7. El IVUS es una herramienta complementaria muy útil en lesiones venosas profundas obstructivas-compresivas al momento de guiar la terapéutica endovascular7,37-41.
En los casos de SCP asociados a obstrucción de la VCI o SMT se recomienda tratamiento endovascular inicial empleando ATP con stent autoexpandible en VCI y/o vena ilíaca según corresponda. Cuando se confirma que la congestión pélvica es debida a SNC (compresión hemodinámicamente significativa de la VRI por la AMS, sin síntomas asociados), se recomienda con bajo nivel de evidencia27 realizar inicialmente ET de las venas con reflujo, reservando la ATP con stent de VRI para pacientes con hematuria, dolor lumbar severo o várices persistentes después de la ET7,18,30-37. En la actualidad es excepcional el empleo del tratamiento quirúrgico vascular convencional de estas lesiones.
4.5.3.1 Obstruction of inferior vena cava and May-Thurner syndrome
Pathophysiology and clinical presentation
MTS is a clinical-anatomical condition in which the left common iliac vein is compressed by the right common iliac artery, or chronic venous endothelial damage is caused by the constant arterial pulsatility with final obstruction. The resulting venous hypertension gradually progresses into venous incompetence, left lower limb swelling, pain, and functional impotence. A total of 6 anatomical variants of MTS have been described and should be taken into consideration because they can compromise both iliac axes (left and right):
1. Compression of the left common iliac vein by the right common iliac artery.
2. Compression of the left common iliac vein by the left internal iliac artery or tortuous and elongated left common iliac artery.
3. Compression of the right common iliac vein by the right common iliac artery.
4. Compression of the right common iliac vein by the right internal iliac artery.
5. Compression of the external iliac vein by the inguinal ligament.
6. Compression of the right common iliac vein by the left common iliac artery in patients with left IVC (29).
In the presence of trigger factors such as hormonal therapy, pregnancy or thrombophilia, some patients can also show extended ilio-femoral and distal forms of acute DVT even with phlegmasia (29,45).
The degree of IVC obstruction, whether acute or chronic, can be very different. It often associates unilateral or bilateral long-term iliac venous obstruction and various symptoms due to the common development of an efficient collaterality. Causes include IVC developmental anomalies (hypoplasia, agenesis, membranes), the Budd-Chiari syndrome, MTS affecting the IVC, postoperative problems (use of IVC filters included), the post-thrombotic syndrome (use of central catheters included), idiopathic issues, malignant tumors, thrombophilia, adenopathies, and adventitial cyst (42,43).
Collateral venous drainage of MTS and IVC obstructions is performed through some of these main circuits: ilio-lumbar veins, perivertebral veins, the hemiazygos-azygos system, pelvic and abdominal parietal veins, and through the hypogastric vein circuit that runs through the presacral plexus and into the IVC. If this hypogastric collaterality is performed through the utero-ovarian and gonadal veins, the obstructive development of PCS can occur (44).
In many of these patients it is possible to identify the PFL into LL by just looking at atypical varicose veins in relation to pudendal, gluteal, obturator or inguinal points.
Although the incidence rate of MTS is still under discussion, it is prevalent in young women from 20 to 40 years of age. Approximately 80% of the patients with MTS develop ilio-femoral DVT and 20% develop venous incompetence (45).
Technique
Beyond etiology, the treatment of choice to re-establish the normal antegrade venous flow in MTS and IVC obstruction is to perform a PTCA with stenting (44-49). Thus, the management of PCS associated with these causes is also a PTCA with stenting because it improves symptoms significantly, quality of life, and even ovarian vein incompetence (6,32,33). The TE therapy of ovarian vein reflux is spared for cases of persistent symptoms post-PTCA. Nevertheless, according to some authors, in women with MTS associated with ovarian vein reflux and a significant variceal load at pelvic level, simultaneous treatment is advised (6,30,31).
The use of venous access to perform these PTCAs varies depending on the region and spread of the target venous lesion. The right internal jugular vein access is perfect to treat IVC obstructions and the common femoral vein access is suitable to treat significant MTS related stenosis. However, for the management of extensive ilio-femoral or iliocaval thrombotic obstructions multiple venous accesses may be necessary including the superficial femoral, popliteal or tibial veins. That is why there is not such thing as a one-size-fits all approach for all the cases. Instead, proper training, ultrasound-guided catheterizations, and strategy planning are necessary to be able to face damaged anatomies (29-32).
Guidewires of different characteristics combined with hydrophilic-coated support catheters are used to recanalize extensive and chronic obstructions of the IVC and iliac veins, both acute and chronic. In case of a recent thrombosis, the post-recanalization is completed by fragmentation, thrombus aspiration, and the occasional infusion of thrombolytic agents in situ (pharmacomechanical thrombolysis). Some heart teams use additional removable vena cava filters during the management of acute thrombosis. Afterwards, support metal guidewires (eg, the Amplatz Super Stiff) and large-diameter high-pressure balloons (usually 10 mm to 24 mm) allow us to dilate the IVC or iliac vein obstructed segment prior to stenting. Both the spread of the lesion and the proximal-distal landing zones for stent implantation should be determined accurately, if possible, under IVUS guidance. Self-expandable large-bore nitinol tents are often used (20 mm to 22 mm for the IVC and 14 mm to 18 mm for the iliac veins) with variable lengths (from 40 mm to 150 mm).42-44 (Figure 11).
Phlebography and pressure measurements should be able to identify iliac, femoral vein or IVC stenoses. Nonetheless, the IVUS is superior to phlebography for the detection of significant stenosis (7,48). Also, the IVUS identifies the proximal implant site in the iliocaval confluence with greater accuracy prior to stent implantation. Afterwards, during post-PTCA assessment, it also allows us to determine the position and configuration of the stent with great accuracy (7,38-41,48).
Together with the common iliac vein, the accurate assessment of the entire IVC, iliac and femoral veins with IVUS is advised to detect any additional obstructions at entry or exit levels that could affect the patency of the stent (7).
Predilatation of iliac femoral or IVC venous stenoses with balloons of proper diameters is mandatory and should always be prior to stenting because it facilitates its correct implantation by solving the fibrotic adherences that are so typical of MTS.
The Society of Vascular Surgery and the American Venous Forum recommend self-expandable stents to treat MTS-type iliocaval compression syndromes (29,49). Currently, the most commonly used onesare the self-expandable nitinol stent that is between 14 mm and 18 mm in diameter and for venous use only. The correct positioning of the stent should exceed the compression of the left iliac vein by right iliac artery and slightly protrude into the IVC (6,7,18,50,51). The new venous nitinol stents designed with greater radial strength facilitate opening and overcome the double force exerted by venous retraction and iliac arterial pressure (in MTS) while keeping the proper flexibility to “copy” the venous anatomy. Of precise positioning and delivery, there is no room for significant shortening. For this reason, lengths should be selected based on the spread of the lesion to be covered (available measurements: from 40 mm to 150 mm). Some specially designed stent models come with reinforced proximal borders and meshes with wide open cells to offer less resistance to contralateral iliac vein flow (52). However, the superiority of the stents available today in the long-term patency of the iliac vein has not been confirmed yet (7).
Postdilatation after stent implantation is still important for the complete expansion of the stent andprevent stent migration.
A control phlebography post-PTCA should confirm the correct positioning of the stent, the proper venous inflow and outflow, and the resolution of the iliac, femoral or IVC occlusion with disappearance of collateral drainage circuits (6,18).
We recommend the combined therapy of all pelvic leaks due to obstructive or compression syndrome because, in most patients, sustained venous hypertension causes the definitive claudication of the valvular system with persistent refluxes after the PTCA. The TE of these incompetent pelvic veins using the sandwich technique with sclerosing foam and controlled delivery coils gives excellent results.
Results
Endovascular recanalization using PTCA with self-expandable stents and balloons has proven to be a safe and durable procedure in cases of both acute and chronic stenosis and/or occlusion of the iliac vein and the IVC (42-44,46,47). With technical success rates over 94%, the iliac venous primary and secondary patency was maintained at the 5- and 7-year mark in non-thrombotic obstructions, acute thrombotic obstructions, and chronic obstructions in 96% to 99%, 87% to 89%, and 79% to 94%, respectively (42-54).
With acceptable reported mortality rates (0.1% to 0.7%), this venous revascularization can become complicated with early re-thrombosis in 1% to 6.8% of the cases, bleeding (0.3% to 1.1%), and PTE (0.2% to 0.9%) on rare occasions (46).
Although there is no undisputed evidence on the use of anticoagulant therapy after PTCA with stenting in veins, the general consensus is to administer anticoagulant therapy for 6 months if there is no formal contraindication or higher bleeding risk. The use of antiplatelet therapy is left at the discretion of each medical team and also based on the need for antiplatelet therapy due to other medical indication (53,54).
Healthcare measures include the use of elastic compression of the damaged limb, occasional help from venoactive drugs, clinical follow-up (VAS and QOL), and control with diagnostic imaging modalities across the years.
(See the Imaging Diagnosis and Surgical Treatment chapters for further information).
4.5.3.2 The nutcracker syndrome
Phenomenon, syndrome and differential diagnosis
The actual incidence rate of the nutcracker syndrome (NCS) is unknown, but in general, it is a relatively rare entity often misdiagnosed as a cause of PCS or the typical NCS symptoms.
We should distinguish the nutcracker syndrome from the nutcracker phenomenon. In the syndrome, compression of the LRV by the SMA is associated with the presence of symptoms, usually lower back pain, PCS, proteinuria, and left renal micro- or macrohematuria. In the phenomenon, the compression of the LRV is not accompanied by the typical symptoms of PCS (7).
Although the assessment of suspected LRV compression is initially performed noninvasively througha CDE, a CAT scan or an MRI, diagnosis is confirmed through renal phlebography and measurement of the renocaval venous pressure gradient or through the IVUS. Both diagnostic imaging modalities, though invasive, are still the gold standard (41).
Despite the high technical accuracy of these imaging modalities, the range of renocaval pressure gradients between patients with NCS and healthy people can overlap. This is not only due to the fact that there is such thing as a range of pressure gradients in asymptomatic patients, but also to the fact that patients with NCS can have “normal” pressure gradients (< 3 mmHg). This can be explained by the compensation of renal venous hypertension that sparks the development of collateral circulation, through the lumbar or gonadal veins (41), that occurs in some instances of the nutcracker phenomenon where the gonadal vein collateral dilatation and drainage can cause symptoms of PCS without the typical NCS symptoms (7). On the other hand, although the IVUS has a greater specificity compared to phlebography for the diagnosis of deep vein stenoses and thromboses (7,41,48), a significant stenosis confirmed through this imaging modality (> 50% of the LRV lumen) does not necessarily mean that we are in the presence of a clinically relevant stenosis (7).
Gonadal and iliac dynamic phlebography (GIDP) associated with the use of the Valsalva maneuver is a valuable tool to determine the presence of LRV compression in its anterior or posterior variants, and to identify collateral venous drainage and flow direction. Also, to distinguish primary from obstructive gonadal vein incompetence due to the NCS.
The Ibero-American Working Group of Pelvic Studies is currently conducting a prospective and retrospective study of hemodynamic assessment in the NCS setting. They intend to identify different patterns of the bypass pathways that may be associated with the clinical signs in order to guide the therapies. The protocol suggests the use of left renal phlebography, sustained Valsalva maneuver, and 360-degree rotational image acquisition of the patient.
The differential diagnosis of the NCS should be performed with renal lithiasis, glomerulonephritis, endometriosis, renal vascular malformation, varicocele, musculoskeletal disorders with left lower back pain, and PCS due to primary venous incompetence (29).
Treatment
After the presence of the nutcracker phenomenon or syndrome has been confirmed, a thorough assessment of the clinical, laboratory, and imaging findings should be conducted to better individualize the therapies. Although there are several therapeutic strategies for the management of the NCS, to this date, no consensus has been achieved on the treatment technique to be used with evidence of good quality and recommendation of great efficacy and safety for most cases (29,37).
In patients with mild and/or transient symptoms of hematuria and lower back pain or in patients under 18, early clinical therapy is advised. Both weight gain and medication to improve renal perfusion can achieve long-lasting improvements in 30% to 68% of the cases (37).
(The details and results of the open and laparoscopic surgical treatment will be discussed in the chapter devoted to vascular surgery).
Over the last few years, several studies have been published on the growing experience with the endovascular management of the NCS with PTCA plus stenting favored by the development of venous PTCA in other territories with excellent benefits and low long-term morbidity and mortality (32-37).
In the strategic planning of PCS cases associated with the “nutcracker phenomenon” and signs of gonadal vein incompetence, an early TE of the gonadal and/or pelvic veins with reflux and leak points into the LL is advised. However, in the presence of the NCS plus PCS with hemodynamically significant stenosis of the LRV (as confirmed by a higher renocaval pressure gradient or spontaneous LRV reflux into the gonadal vein), a PTCA with stenting on the LRV is suggested initially.7,23,36,37
It is advisable to perform the combined therapy of left gonadal reflux with TE associated with the PTCA with stenting on the LRV because the PTCA alone cannot fix secondary pelvic congestions (36). A subsequent gonadal TE treatment can become difficult because of the presence of the previous stent and gonadal vein proximal thrombosis.
Technique
The PTCA with stenting on the LRV is a technically complex procedure that requires specific technical training and proper knowledge of the materials needed.
Access via internal jugular vein is usually more appropriate because it provides good support, control, and accuracy to later use the material needed for the catherization and the PTCA. In cases of horizontal LRV, femoral access can also be a good option, and double jugular and femoral access is always advised. The use of an Amplatz stiff guidewire with a short, flexible tip followed by a 50cm long vascular introducer sheath provides support and control when performing a PTCA on the LRV. Self-expandable nitinol stents of 12 mm to 16 mm in diameter and 40 mm to 60 mm in length are often used (the main experience published to this date used dedicated non-venous self-expandable stents) (32-37) (Figures 12, and 13). Authors with over 20 years of experience performing endovascular procedures to treat PCS and compression syndromes like Dr. Zubicoa and Dr. Leal Monedero have witnessed the advances made on the different materials used: coils, plugs, and stents. They claim that these nitinol stents specifically designed for venous disease are the most suitable ones (18,36).
Nonetheless, the delivery of the Wallstent endoprosthesis stent can be aborted once it has begun and repositioned if implanted incorrectly. This great advantage added to the operators’ experience with minimal complications make it the perfect choice for the management of the NCS (36) (Figures 12, and 13).
We should mention that the Wallstent endoprosthesis stent was one of the first stents ever used to treat venous disease by Dr. Neglen and Dr. Raju (both experienced using this stent) (38,40,43,45).
The diameter of the stent should be oversized by 20% in the LRV. Also, predilatation with a 14 mm to 16 mm balloon is recommended before stent implantation. However, some authors avoid using predilatation on a routine basis. According to these authors, the incidence rate of LRV synechiae or thrombosis due to the NCS is rare (36) (Figure 14).
When necessary, in-stent postdilatationshould be carefully performed to avoid altering the integrity or positioning of the stent.7 The proximal border of the stent implanted should run parallel to the LRV ostium to avoid its protrusion into the IVC (6,18).
Pelvic and gonadal venous leaks and hypogastric branches due to the NCS should be treated with TE. The combined therapy of gonadal TE and the sandwich technique associated with the PTCA with stenting is advised to avoid incomplete or insufficient treatments (36) In most cases, secondary gonadal reflux persists due to the presence of residual valvular incompetence.
The control renal phlebography post-PTCA should confirm the washout into the ICV and the absence of ovarian venous reflux or into other perirenal veins, as well as the normalization of the renocaval pressure gradient from 0 to 1 mmHg as indicators of a successful therapy (6,7,18,34,36). However, some studies published claim that this normalization of the post-PTCA or surgical pressure gradient inexplicably did not follow the clinical resolution achieved (37).
On the one hand, follow-up should be performed by quantifying to what extent lower back pain improved while looking for signs of PCS, if present, and controlling hematuria. On the other hand, the images obtained through the CDE and CAT scan and/or the MRI should beused to confirm the success of the procedure and tip off about the main risk of stent migration.
Results
The series published of PTCA for the management of the NCS, though heterogeneous in the number of cases, follow-up time, type of stent used, and imaging modality reported, reveal interesting clinical success rates with variable improvements in 80% to 96% of the cases.
However, the rate of major complications of occlusion, stent fracture but, above all, stent migration to the heart chambers is between 0% and 6% according to the different studies published so far. This is indicative of the need to accurately adjust the indication of PTCA compared to other therapies and have the necessary and specific experience (29,32-37). The development of a specific stent could prevent these parietal fixation problems. For this reason, the PTCA for the management of the NCS has a Recommendation Class II or lowand aLevel of Evidence C.
(See the chapters on Imaging Diagnosis and Surgical Treatment for additional information)
4.5.4 Recommendations
|
1 |
We recommend the endovascular treatment of PCS especially in young women. (Level of Evidence B, Recommendation Class I) (6,29 ). The TE is recommended as the treatment of choice for the management of PVR of nonobstructive causes. Venous PTCA with stenting is advised as the early treatment of choice for the management of obstructive PCS (6,7). |
|
2 |
Emplear de preferencia la técnica de ET en sándwich de venas gonadales6-18 y ramas hipogástricas con fugas de piso pelviano. |
|
3 |
En ET emplear coils fibrados de liberación controlada largos para tratar venas gonadales insuficientes hasta el sector más proximal. |
|
4 |
Tratar con ET todas las fuentes de reflujo pélvico-abdominal hacia MMII para minimizar la recurrencia de síntomas5-14. |
|
5 |
En SCP por obstrucción de VCI o SMT, se recomienda resolución del fenómeno compresivo (ATP) asociado al tratamiento simultáneo de la congestión pélvica secundaria (ET) responsable de la sintomatología. (Nivel de Evidencia B, Recomendación Clase I-II) 6,7,18,29-37. |
|
6 |
En el SNC actualmente está discutido la resolución endovascular o cirugía abierta. Recomendamos emplear procedimientos endovasculares combinados del reflujo gonadal (ET) y ATP de VRI con stent por la mínima morbilidad y elevada eficacia. (Nivel de Evidencia B, Recomendación Clase IIa)7,18,29-37. |
|
7 |
Emplear IVUS en SCP asociado a SNC y/o SMT tanto para la confirmación diagnóstica como para guiar el procedimiento terapéutico7,38-41,48. |
|
8 |
Recomendamos realizar consultas clínicas de control a los 3 y 6 meses empleando índices (VAS, QOL) posprocedimiento con evaluación de EDC y RM de control 6,10,16. |
4.5.5 References
- Reginald PW, Kooner JS, Samarage SU et al. Intravenous dihydroergotamine to relieve pelvic congestion with pain in young women. Lancet 1987;330:351–3.
- Edwards RD, Robertson IR, MacLean AB, et al. Case report: Pelvic pain syndrome—successful treatment of a case by ovarian vein embolization. Clin Radiol 1993; 47:429–431.
- Borghi C, Dell’Atti L. Pelvic congestion syndrome: the current state of the literature. Arch Gynecol Obstet. 2016;293(2):291-301.
- Brown CL, Rizer M, Alexander R, et al. Pelvic Congestion Syndrome: Systematic Review of Treatment Success. Semin Intervent Radiol 2018;35:35–40.
- Asciutto G, Asciutto KC, Mumme A, Geier B. Pelvic venous incompetence: reflux patterns and treatment results. Eur J Vasc Endovasc Surg 2009;38:381-6.
- Dándolo MA; Paolini JE. Tratamiento del síndrome de congestión pélvica. Tratamiento endovascular. En:Simkin R, et al. (eds) Guías latinoamericanas de terapeútica para la patología venosa: Insuficiencia Venosa Crónica, (IVC), 1er Edición. Nayarit-Buenos Aires Argentina, 2016, pp 304-310.
- Antignani PL, Lazarashvili Z, Monedero JL. Diagnosis and treatment of pelvic congestion syndrome: UIP consensus document. Int Angiol. 2019 Aug;38(4):265-283.
- Gandini R, Konda D, Abrignani Set al. Treatment of symptomatic high-flow female varicoceles with stop-flow foam sclerotherapy. Cardiovasc Intervent Radiol 2014;37(5):1259–1267.
- Andrews RT. Pelvic Congestion Syndrome. En: Geschwind J, Dake M (eds) Abrams’ Angiography: Interventional Radiology, 3rd edition.Philadelphia, PA: Lippincott Williams & Wilkins,2013, pp. 278-290.
- Boyer L, Maubon A, Ravel A, Gageanu C, et al. Insuffisance veineuse pelvi-périnéale. En: Chabrot P, Boyer L (eds) Embolisation. Springer-Verlag France, 2012, pp 333-346.
- Lopez AJ. Female pelvic vein embolization: indications, techniques, and outcomes. Cardiovasc Intervent Radiol 2015; 38(4):806–820.
- Ratnam LA, Marsh P, Holdstock JM, et al. Pelvic Vein Embolisation in the Management of Varicose Veins. Cardiovasc Intervent Radiol 2008; 31:1159–1164.
- Kim HS, Malhotra AD, Rowe PC, et al. Embolotherapy for pelvic congestion syndrome: long-term results. J Vasc Interv Radiol 2006; 17:289–297.
- Venbrux AC, Chang AH, Kim HS, et al. Pelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain. J Vasc Interv Radiol 2002; 13:171–178.
- Kwon SH, Oh JH, Ko KR, et al. Transcatheter Ovarian Vein Embolization Using Coils for the Treatment of Pelvic Congestion Syndrome. Cardiovasc Intervent Radiol 2007; 30:655–661.
- Black CM, Thorpe K, Venrbux A, et al. Research Reporting Standards for Endovascular Treatment of Pelvic Venous Insufficiency. J Vasc Interv Radiol 2010; 21:796–803.
- Hocquelet A, Le Bras Y, Balian E, et al. Evaluation of the efficacy of endovascular treatment of pelvic congestion syndrome. Diagnostic and Interventional Imaging 2014 95, 301—306.
- Leal Monedero J. Indicaciones y tratamiento del síndrome de congestión pélvica. Rev de Flebología y Linfol. Lecturas vasculares. 2010. 14:841-47.
- Ganeshan A, Upponi S, Hon L-Q, et al. Chronic Pelvic Pain due to Pelvic Congestion Syndrome: The Role of Diagnostic and Interventional Radiology. Cardiovasc Intervent Radiol. 2007; 30:1105–1111.
- Bittles MA, Hoffer EK. Gonadal Vein Embolization: Treatment of Varicocele and Pelvic Congestion Syndrome. Semin Intervent Radiol. 2008;25:261–270.
- Guirola JA, Sanchez-Ballestin M, Sierre S, et al. A randomized trial of endovascular embolization treatment in pelvic congestion syndrome: fibered platinum coils versus vascular plugs with 1-year clinical outcomes. J Vasc Interv Radiol. 2018;29:45-53.
- Jarrell JF, Vilos GA, Allaire C, et al.No. 164-Consensus Guidelines for the Management of Chronic Pelvic Pain. J Obstet Gynaecol Can 2018;40(11):747−787.
- Chung M-H, Huh C-Y. Comparison of treatments for pelvic congestion syndrome. Tohoku J Exp Med. 2003;201:131–138.
- Daniels JP, Champaneria R, Shah L, et al. Effectiveness of embolization or sclerotherapy of pelvic veins for reducing chronic pelvic pain: a systematic review. J Vasc Interv Radiol 2016; 27:1478–1486.
- Mahmoud O, Vikatmaa P, Aho P, et al. Efficacy of endovascular treatment for pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord 2016; 4:355–370.
- Champaneria R, Shah L, Moss J, et al. The relationship between pelvic vein incompetence and chronic pelvic pain in women: systematic reviews of diagnosis and treatment effectiveness. Health Technol Assess 2016; 20:1–108.
- Khilnani N, Meissner M, Learman L, et al. Research priorities in pelvic venous disorders in women: Recommendations from a multidisciplinary research consensus panel. J Vasc Interv Radiol 2019; 30: 781–789.
- Hansrani V, Abbas A, Bhandari S, Caress AL, Seif M, and McCollum CN. Trans-venous occlusion of incompetent pelvic veins for chronic pelvic pain in women: A systematic review. Eur J Obstet Gynecol Reprod Biol 2015;185:156–63.
- Gloviczki P. Guidelines 5.7.0 American Venous Forum on the management of pelvic congestion and perineal varicosities. p:695,811. In Handbook of Venous and Lymphatic Disorders. 4th Edition 2017. CRC Press.Taylor and Francis Group. ISBN 13:978-1-4987-2440-1.
- S. F. Daugherty and D. L. Gillespie. Venous angioplasty and stenting improve pelvic congestion syndrome caused by venous outfow obstruction. J Vasc Surg: Venous and Lym Dis 2015; 3:283-289.
- Santoshi RKN, Lakhanpal S, Satwah V, et al. Iliac vein stenosis is an underdiagnosed cause of pelvic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2017;6:202-11.
- d’Archambeau O, Maes M, De Schepper AM. The pelvic congestion syndrome: role of the “nutcracker phenomenon” and results of endovascular treatment. JBR-BTR. 2004;87:1-8.
- Scultetus A, Villavicencio J, Gillespie D. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg 2001;34:812-9.
- Hartung O, Grisoli D, Boufi M, Marani I, Hakam Z, Barthelemy P, et al. Endovascular stenting in the treatment of pelvic congestion syndrome caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg 2005;42:275-80.
- Berthelot JM, Douane F, Maugars Y, Frampas E. Nutcracker syndrome: a rare cause of left flank pain that can also manifest as unexplained pelvic pain. Joint Bone Spine. 2017;84(5):557–62.
- Leal Monedero J, Zubicoa Ezpeleta S, Perrin M. Management of left renal vein compression in patients presenting left gonadal vein reflux. Phlebology. 2018;33(7):475-482.
- Velasquez CA, Saeyeldin A, MD, Zafar MA et al. A systematic review on management of nutcracker syndrome. J Vasc Surg: Venous & Lym Dis 2018;6:271-8.
- Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg 2002;35:694–700.
- Labropoulos N, Jasinski PT, Adrahtas D, Gasparis AP, Meissner MH. A standardized ultrasound approach to pelvic congestion syndrome. Phlebology 2017;32:608–19.
- Raju S, Montminy ML, Thomasson JD, et al. A comparison between intravascular ultrasound and venography in identifying key parameters essential for iliac vein stenting. J Vasc Surg: Venous and Lym Dis 2019;-:1-7.
- Ananthan K, Onida S, and Davies AH. Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. 2016; 53(6):886–894.
- Erben Y, Bjarnason H, Oladottir GL, McBane RD, Gloviczki P. Endovascular recanalization for nonmalignant obstruction of the inferior vena cava. J Vasc Surg Venous Lymphat Disord 2018. pii: S2213-333X(17) 30533-4.
- Raju S, Hollis K, Neglén P. Obstructive lesions of the inferior vena cava: Clinical features and endovenous treatment J Vasc Surg 2006;44:820-7.
- E.H. Murphy, B. Johns, E. Varney, S. Raju. Endovascular management of chronic total occlusions of the inferior vena cava and iliac veins. J Vasc Surg Venous Lymphat Disord, 5 (1) (2017), pp. 47-59.
- Neglén P, Thrasher TL, Raju S. Venous outflow obstruction: an underestimated contributor to chronic venous disease. Journal of Vascular Surgery 2003; 38: 879 – 885.
- Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv 2015; 8:e002772.
- Neglén P, Hollis KC,Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007;46:979-90.
- Gagne PJ, Tahara RW, Fastabend CP, Dzieciuchowicz L, Marston W, Vedantham S, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord 2017;5:678–87.
- Meissner MH, Gloviczki P, Comerota AJ, et al. Early thrombus removal strategies for acute deep venous thrombosis: Clinical Practice Guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012; 55:1449–1462.
- Raju S, Ward M Jr, Kirk O. A modification of iliac vein stent technique. Ann Vasc Surg. 2014; 28:1485–1492.
- Le TB, Lee TK, Park KM, Jeon YS, Hong KC, Cho SG. Contralateral deep vein thrombosis after iliac vein stent placement in patients with May- Thurner syndrome. J Vasc Interv Radiol 2018; 29:774–780.
- De Wolf MAF, De Graaf R, Kurstjens RLM, Penninx S, Jalaie H, Wittens CHA. Short-term clinical experience with a dedicated venous nitinol stent: initial results with the sinus-venous stent. Eur J Vasc Endovasc Surg 2015; 50:518–526.
- Carroll S, Moll S. Inferior vena cava filters, May-Thurner syndrome, and vein stents. Circulation. 2016;133(6):e383–7.
- Goldman RE, Arendt VA, Kothary N, et al. Endovascular management of May-Thurner syndrome in adolescents: a single center experience. J Vasc Interv Radiol. 2017; 28(1):71–7.
4.6 Surgical treatment
Authors: Oscar Gural Romero, Santiago Gil, Eugenio Piraino
4.6.1 Introduction
The therapeutic management of PCS is complex if we take into account that it involves young patients, mostly in their fertile years with symptoms not always attributed to venous incompetence, but to gynecological, urological, traumatic, and even psychological disorders.
The use of psychotherapy, hormonal therapy, progestogens and gonadotropin receptor antagonists has been described with uncertain and limited results (1).
Thanks to the knowledge provided by pathophysiology and use of additional imaging modalities like the CDE, the GIDP, the CAT scan, and the MRI, gonadal and hypogastric axis reflux has been established as the origin of symptoms either in isolation or in association with compression or post-thrombotic syndromes.
Given the lack of results from the symptomatic or hormonal medical therapy, surgical treatments have described for over 22 years including selective venous ligations and anexo-hysterectomies. Conventional or robot-guided laparoscopic approaches have been developed for gonadal vein ligation. Although the early results on the management of PCS were optimal, complications were reportedly associated with open surgery and with a high rate of symptom persistence and relapse. This ignited the search for minimally invasive endovascular procedures. The TE has had a crucial evolution over the last few years and has yielded excellent results with less morbidity and relapses and fast patient recoveries. That is why, to this date, it is considered as the gold standard. We will be analyzing the different surgical options below including their indications, limitations, complications, and long-term results. Each with its own precise indication within the current therapeutic armamentarium of PCS (2).
We can categorize them into conservative (or open) surgical procedures and non-conservative or minimally invasive ones.
4.6.2 Conservative surgical treatment
6.6.2.1 Selective gonadal vein ligation through open or conventional approach
Intra- or extraperitoneal gonadal vein ligation is one of the most commonly used techniques. Back in 1984, Rundqvist et al. described ovarian vein extraperitoneal resection to treat PCS. A total of 70% of the patients got better at the short-term follow-up (3,4)
Back in 1985, Lechter et al. introduced the technique of gonadal vein ligation via open surgery. Treatment was administered on the most significant side, and bilaterally in some cases. The objective of this technique is to avoid the reflux and the PVR caused by gonadal veins without compromising the distal treatment of these veins that are responsible for symptom persistence or varicose vein relapse (5).
The drawbacks of these open procedures (prolonged ileus, infections, hematomas, and intestinal bridles) are directly associated with the approach described.
4.6.2.2 Laparoscopic selective gonadal vein ligation
The arrival of laparoscopy in the diagnosis of CPP played an important role in its etiological and therapeutic definition. With a 77% specificity and a 96% sensitivity and associated with the clinical signs of pelvic floor pain, dyspareunia, vulvar or atypical varicose veins, it certifies the diagnosis of PCS (Figure 15).
There are former studies on ovarian and other pelvic vein ligation via extraperitoneal and transperitoneal access through laparotomy or laparoscopy. Rates of improvement over 70% are reported. However, most of these studies are observational and case series with methodological deficiencies. This technique should only be indicated when endovascular procedures fail or areunavailable (3).
Back in 1995, Mathis et al. described the performance of diagnostic laparoscopies associated with selective gonadal ligation (6). In 1998, Gómez et al. published the satisfactory results of 25 patients with CPP and PCS with gonadal ligation (7). Regarding technical details, the laparoscopic approach requires 3 introducer sheaths, creation of pneumoperitoneum, and general anesthesia in a surgical setting. After the corresponding examination and upon confirmation of venous congestion, the retroperitoneal plane is dissected, and the gonadal packet can be interrupted with knots, endosutures, metal clips, bipolar or laser thermal ablation or with the Ligasure technique, all with early satisfactory results (8).
In 2003, Gargiulo et al. introduced their experience with laparoscopic ovarian transperitoneal ligation in 23 women with a 1-year follow-up and a 60-minute surgery under general anesthesia. Pain and varicocele improved significantly in the 23 cases. We should mention that these patients had longer hospital stays and recovery periods and needed general anesthesia compared to patients treated with the endovascular techniques. Follow-up times were short considering that most relapses occur after 2 years (9).
Ovarian vein ligation gives immediate satisfactory results but is also associated with comorbidities and longer hospital stays (10) (Table 1).
We should mention complications like vascular or organ lesions caused by trocars, gonadal vein ruptures, and clinical signs attributed to the creation of pneumoperitoneum.
In their multicenter study, Chapron et al. assessed 29966 laparoscopies with a rate of complications of 4.6% for every 1000 procedures and one death associated with this procedure. Most complications were due to trocars and to the creation of pneumoperitoneum being the human factor (the operator) a crucial factor here. According to different studies conducted, the rate of complications is between 2% and 5% for every 1000 procedures (11-13).
Laparoscopy (often used for the diagnosis and treatment of other causes of CPP such as endometriosis and adherences) is, however, ineffective to diagnose PVR. CO2 pressure and the Trendelemburg position can collapse dilated pelvic veins and they can go misdiagnosed. Since we other non-invasive diagnostic imaging modalities are available, the laparoscopy should not be used as the first-line therapy to rule out the presence of suspected PCS.
Complications of laparoscopic ligation (14-16)
• Lesions due to trocar insertion: 19% were major lesions that required conversion to open surgery. Vascular, intestinal, and urinary lesions occurred in 80%, 30%, and 25% of the cases, respectively. Most cases were due to technical failures, use of additional approaches, previous history of surgeries, and lack of experience managing complex cases. Currently, techniques with mini-trocars and minicameras have been developed, and even hybrid approaches with mini-laparotomies that reduce the incidence rate of the lesions described above.
• Vascular lesions: they represent 1/1000 of all complications reported including lesions located in the aorta, arteries, iliac veins, vena cava, hypogastric, and gonadal veins. Most of them were caused while trocar insertion. They can be mild or cause life-threatening bleeding. Some can be identified by bleeding coming out of the trocars, but most of them cause retroperitoneal hematomas that can go misdiagnosed and increase when the creation of pneumoperitoneum is interrupted. Monitoring the patient plays an important role here since sustained hypotension, tachycardia, and a situation that does not respond to expansion leads to suspecting the presence of a severe lesion that requires immediate conversion and vascular repair (19).
• Pulmonary thromboembolism due to carbon dioxide can be caused by infusion with a needle at pneumoperitoneum gas IV level and is associated with a high mortality rate. It requires an early participation by the anesthesiologist and the heart team (20).
• Cardiopulmonary complications like arrhythmias, cardiopulmonary shock, and cardiac arrest, often associated with hypercapnia or anoxia, hypoventilation, many of which are dueto a prolonged Trendelemburg position and an inadequate pneumoperitoneum.
• Neurological complications such as brachial femoral neuropathy attributed to unhealthy and prolonged positions and postures (21).
• Intestinal complications: with an incidence rate from 0.03% to 0.6%, they include perforation or thermal injury of colon, small intestine, and stomach. The presence of an inadequate pneumoperitoneum or previous adherences are a predisposing factor. Many of them can go misdiagnosed and later progress into peritonitis and sepsis (22).
• Urological lesions: with an incidence rate that from 0.42% to 1.6%, they include the accidental ureteral C-section or thermal injury and vesical perforations (23).
Limitations of gonadal laparoscopic ligation (24)
• It requires general anesthesia
• It has greater morbidity compared to TE endovascular procedures.
• Vascular, intestinal, neurological, and cardiac lesions are the most significant and severe of all.
• Hospital stays of 2 or more days whereas TE is an outpatient procedure or requires a 12-hour hospital stay in most cases.
• Impossibility to estimate accurately the severity and spread of venous incompetence because of the supine position and vascular venous collapse caused by the pneumoperitoneum.
• Impossibility to assess bypass pathways like the presacral, hypogastric, periovarian or periuterine ones.
• It only allows us to treat gonadal incompetence. It simply cannot solve the incompetence of hypogastric vein branches like the internal pudendal, inferior gluteal, and obturator ones that is responsible for 30% to 40% of pelvic bypass reflux or flow.
• Once the gonadal vein has been obliterated, the possible relapses that may occur cannot be treated.
4.6.3 Non-conservative surgical treatment
4.6.3.1 Hysterectomy and salpingo-oophorectomy
If medical treatment proves unsatisfactory, the hysterectomy, whether associated or not with unilateral or bilateral oophorectomy, can be an option. The patients from different studies have shown early symptom relief. However, subsequent follow-up has revealed the existence of persistent pelvic pain in 33% of the cases and a recurrence of the clinical signs in 20% of the cases associated with the appearance of urinary, rectal, and gynecological disorders (25).
Hartmann et al. conducted a study on quality of life taking into account pelvic pain and depression. They included 1249 women treated with hysterectomy who were followed for 2 years. They initially confirmed a drop in the rate of pelvic congestion from 97% to 19% followed by an improved quality of life (both physical and social) in 50% the patients. The rate of depressive signs dropped from 85% to 33%, and dyspareunia improved in all the patients (26).
Based on their own experience Nieber et al. described that hysterectomy has better results compared to medical and surgical therapy regarding uterine preservation.
Most studies are about conventional hysterectomy with open surgery, but the most recent series show a better quality of life with laparoscopic compared to open hysterectomy at a 4-year follow-up (27,28).
In a comparative study between hysterectomy plus bilateral or unilateral oophorectomy and TE plus hysterectomy followed by hormonal therapy, Chung et al., confirmed that all patients improved significantly. Still, they recommend the TE over open surgery because of the fast recovery times and an outpatient procedure with immediate results. Even diagnostic phlebography can be used as a therapy during the same surgical act, which avoids the use of two radiological procedures (29).
The long-term follow-up of some series confirmed that clinical results are variable, and that symptom and sign improvement is poor and non-significant (29,30)
Symptom persistence or relapse occur due the numerous anastomoses among ovarian, hypogastric, and uterine veins. Salpingo-hysterectomy is not always accompanied by the ligation of these utero-ovarian connections or associated with the treatment of pelvic leak points that are responsible for the clinical signs and the presence of genital or atypical varicose veins in the LL (31).
Table 2 shows the results of the four main series: open techniques (hysterectomy associated with salpingo-oophorectomy) and laparoscopic techniques. Not all studies defined exactly the criteria of pelvic congestion or used pain and patient satisfaction scales. There were no precise data on the results of hysterectomy although early improvement was reported (32).
Hysterectomy can be useful as the last recourse of the therapeutic algorithm in cases of central uterine pain, vaginal fornix dyspareunia, dysmenorrhea, and metrorrhagia unresponsive to standard therapies in patients out of their reproductive years with completed fertility and when endovascular techniques are unavailable or have failed. These surgical techniques have the same results as endovascular therapies but also a higher rate of complications. Therefore, they should not be considered the first-line therapy for the management of PCS (33-35).
4.3.6.2 Complications of open surgery (36)
• Infections. Categorized as infections and often associated with the use of the ventilator or venoclysis. On the other hand, late infections can be due to surgical wound infections, pelvic abscesses, infectious thrombophlebitis or urinary infections. In some cases, they are associated with wound dehiscence such as those located in the abdominal wall and the vaginal vault.
• Intra- and postoperative hemorrhages. They occur due to inadequate hemostasis, release of vascular ligations or vascular diabrosis.
• Digestive such as paralytic ileus, intestinal obstruction or intestinal lesions.
• Dehiscence and evisceration. Revealed by technical defects in the closure of the wall or associated factors such as obesity, COPD, tissue weakness or previous surgeries.
• Thromboembolic complications such as DVT or PTE.
• Urinary. In 70% of the cases, ureteral lesions occur associated with hysterectomy. Vesical lesion is less common.
• Urinary, intestinal or vaginal fistulas.
4.6.4 Vascular and endovascular surgical
treatment of compression syndromes
as the cause of the pelvic congestion
syndrome
In the presence of utero-ovarian varicose veins, pelvic reflux can cause ovarian, uterine, and hypogastric vein incompetence. However, venous hypertension can also be due to venous compression signs. In the NCS and the MTS the compensating bypass pathways cause congestive signs at pelvic level. The DCE is of great diagnostic utility because it can identify pure or mixed congestive-compressive signs (37).
In the assessment of patients with atypical or genital varicose veins, it is of paramount importance to rule out these associated compression syndromes (38).
4.6.4.1 Síndrome de May-Thurner
The May Thurner Syndrome (MTS) is caused by the compression of the left iliac vein by the right iliac artery. However, some anatomical variants like the ones described by Rajú show us that compression can occur at right iliac, hypogastric, bilateral or femoral vein level. This compression triggers venous stasis, the development of bypass pathways with the corresponding high risk of DVT.39
The clinical signs of MTS can be present in 52.2% of the patients with edema, 38% of the patients with pain, 5.9% of the patients with LL ulcers, 52.4% with DVT, 7.8% with PTE, 2.2% with phlegmasia, critical ischemia, compartmental syndrome, 30% with atypical varicose veins and collateral circulation, and 40% of the patients with secondary PCS. A total of 40% of the patients show collateral circulation due to iliac, prepubic, abdominal or intrapelvic obstruction or thrombosis.40,41
We can mention the following radiological criteria: evidence of iliac and femoral obstructions, presacral, lumbar ascending, prepubic collateral circulation; reversed flow by hypogastric veins, periuterine and periovarian pelvic congestion, and appearance of pelvic leak points connected to the LL (Figure 16).
Regarding treatment, most patients have or had signs of DVT whether partially or totally recanalized. Before the year 2000, 75% of all procedures were performed through open surgery while only 25% were endovascular. Bypass techniques were described with the formation of arteriovenous fistulas but with unsatisfactory results due to the high rate of DVT and restenosis.
After the year 2000 this ratio changed, and only 4.1% of the patients were treated with open surgery and 95.9% through endovascular techniques. Recanalizations through catheterization can use thrombolytic drugs (33.2%) or not (53.2%). Anticoagulation and elastic compression therapy are used as the medical treatment (7%) (42-44). Currently, the routine clinical practice is trying the endovascular resolution using a PTCA with balloon and stenting. At the beginning, self-expandable stents were used like the Wallstent endoprosthesis stent (Boston Scientific) because of its adequate diameter eligible for large veins. With the development of venous stents, results have been very satisfactory with high primary and secondary patency (45).
4.6.4.2 The nutcracker syndrome
Pathophysiology and clinical signs
The NCS is due to the compression of the left renal vein by the aorto-mesenteric compass, in the anterior variant or by compression of the aorta and the spine in the posterior variant (46)
This compression causes distal hypertension with left renal and left gonadal vein dilatation, and development of perirenal collaterality. This compression becomes evident on ultrasound, x-ray, and computed tomography findings (Figures 17, 18, 19, and 20).
On the clinical level, patients often complain of hematuria, lower back pain or signs of pelvic congestion. Only those with mild pelvic pain, dyspareunia, and atypical varicose veins, a sign consistent with PCS, are eligible for TE as the early therapy.
If no improvement is reported with the TE and after the gonadal vein has been recanalized, the indication is to proceed with TE again. The occurrence of hematuria, lower back pain (7 on a 10 scale) or renocaval pressure gradients ≥5 mmHg is an indication for renal stent implantation and gonadal TE (47) (Diagram 1).
Therapeutic options of NCS48
1) Conservative treatment
A 24-month wait-and-see approach is expected in women under 18 years and a 6-month wait-and-see approach in adults. In these adult patients, weight gain and the development of collateral circulation improves the clinical signs in 70% of the cases.
The procedure is decided after this period of time or if the following occurs:
• Severe, recurrent hematuria.
• Severe symptoms of flank or abdominal pain.
• Anemia.
• Renal function impairment including persistent orthostatic proteinuria.
• Significant varicocele.
2) Interventional behavior
Podemos clasificarlas como:
• Open surgery.
• Endovascular.
• Laparoscopic.
• Robot-assisted laparoscopy
The earliest surgery studies appeared in 1974 authored by Pastershank et al. In these studies, they described a left renal vein venoclysis (49).
Then they went on to mention different techniques to solve compression and treat the corresponding pelvic congestion (50,51).
• Renal vein transposition.
• Renal vein transposition with patch, cuff, and both.
• Renal autotransplantation.
• Gonadocaval bypass.
• Left renal vein ligation and iliac vein bypass.
Some of the techniques described above are very complex like left renal autotransplantation, and even nephrectomy has been described in extreme cases.
Bypass and decompressive techniques include renal vein transposition, gonadocaval bypass, splenorenal bypass, and renal inferior mesenteric bypass through open surgery or laparoscopy (52,53).
Gonadocaval bypass can be performed with a reduced retroperitoneal approach, does not require renal vein dissection, and has a special indication in cases of retroaortic renal vein. In most patients the gonadal vein appears dilated and elongated, which can be confirmed previously through additional assessments that facilitates the bypass surgery. The simple lateral clamping of the IVC followed by a single anastomosis is a simple technique with a low morbidity rate (54,55).
In our own experience of 3 the cases treated it was decided to go with bypass surgery because patients refused stent implantation. The advantage of this technique is that it does not only achieve an effective bypass pathway, but it can also be combined with other techniques like the distal embolization of the remaining stump with sclerosing foam, which facilitates the simultaneous management of the associated PCS (Figure 20).
Although few papers have been published on this type of procedures, it has been confirmed that left renal vein transposition is more effective and is associated with less morbidity. In a series by Reed et al. where venous transposition was performed, the symptoms and signs of 80% of the cases disappeared at the 12-year follow-up without renal failure and mortality. The following complications have beenreported though: paralytic ileus and retroperitoneal hematoma. Reintervention was unnecessary at the 12- and 24-month follow-up in 76% and 68% of the cases, respectively. The most common cause of symptom relapse is left renal vein restenosis or obstruction, a condition that can be solved through endovascular treatment (56).
Among the criticism made to these procedures we find the need for laparotomy with long hospitalization stays and the associated risks of stenosis during anastomosis, which is why different techniques have been proposed to prevent these complications like the use of patches, cuffs, or both.
Chau et al. introduced their experience with left renal vein transposition through Da Vinci robot-assisted laparoscopy. This technique gives excellent results, but the technology is not widely available and requires specific training, which is why its use is limited to high-complexity health centers.57
Wang et al. presented a series of 13 patients using the laparoscopic extravascular stent placement technique. A 10 mm-diameter-PTFE stent was implanted. The radioscopy and ultrasound control assessment performed confirmed complete symptom resolution in 77% of the cases and partial symptom resolution in 15% of the cases. External stent migration was reported in one case only.58 In 3 patients treated with laparoscopic extravascular stent placement, Zhang et al. did not find any complications either and compression was solved with decreased renal artery velocities and gonadal vein diameter.59
Endovascular resolution requires PTCA and venous stenting with gonadal TE; the first paper on this matter was published by Neste back in 1996.60
It is a minimally invasive option that only allows us to perform endovascular renal stent implantation or combine it with gonadal TE. This endovascular procedure is associated with one of the highest risks of complications or therapeutic failure. The renal vein diameter varies with different postures and during the Valsalva maneuver and anatomical variations like retroaortic renal vein can occur complicating stent cannulation and implantation. To avoid stent migrations, the diameter of the stent should be oversized, we believe, by 30%.
Wu et al. published a series of 75 patients. A total of 5 patients (6.6%) experienced intracaval and atrial stent migration (61).
Chen et al. presented a retrospective study of 61 patients. A total of 96.7% of the patients (59 out of 61) improved pain and hematuria significantly at the 6-month follow-up without restenosis at the 66-month median follow-up. The LRV diameter, peak velocity ratio, and renocaval pressure gradient all improved. They reported two stent migrations, 1 of them into the atrium that required open-heart surgery.62,63
In most series with few cases the authors used undedicated stents to treat veins with a high rate of migration. Currently, however, self-expandable nitinol venous stents are available. They have great radial strength and behave differently from the early stents. The worldwide experience with this type of material is not much, but still encouraging. To this date, there is not such thing as a venous stent specifically designed for the renal vein, which means that dedicated stents are being used in veins from other territories. The stent should protrude slightly from the renal vein into the vena cava and be placed distally towards the renal hilum. Given the high risk of stent displacement or malapposition, some authors combine renal stenting and external fixation with vasorraphy to prevent its mobilization through mini laparotomy.
Secondary pelvic hypertension and collaterality are not always solved with stent implantation alone because of the abundant collaterality and anastomosis present at distal level. Therefore, a comprehensive therapy is advised. Initially, the best thing to do is to perform a selective distal-to-proximal TE of periuterine and adnexal pelvic vessels and left gonadal vein tributaries with coils and foam followed by stent implantation into the renal vein. Here stent implantation would only limit the possibility of using the endovascular gonadal approach eventually later or in the long-term (64).
The endovascular option is the first option over the conventional one because the patient recovers faster, because it is minimally invasive, with less morbidity, and because hospital stays last no more than 24 hours. Also, if it fails, we can repeat procedure or cross over to open surgery
To this point, multicenter studies with long-term follow-up periods should be conducted. However, the medical literature available to this date considers the endovascular option the first-line therapy after conservative medical treatment.
4.6.5 Analysis and considerations
PCS requires the comprehensive assessment of the patient, anatomical and pathophysiological knowledge, and use of all additional tests that may be necessary to identify its origin and future disease progression.
Conservative management with general, pharmacological measures is considered the first-line therapy, but if it proves ineffective or in the presence of symptomatic severe venous congestion, the interventional option can be considered. Conventional surgery with salpingo-oophorectomy-hysterectomy has become the go-to technique with acceptable short-term results. However, morbidity and symptom persistence were still reported in 30% of the series including the appearance of new associated conditions like urinary incontinence and prolapse.
The development of laparoscopy allowed us to reduce the comorbidity associated with open surgery. However, in the case of gonadal vein ligation, it improved symptoms though with a high rate of relapse due to the untreated leaks at pelvic and periuterine-ovarian plexus level.
Less postoperative pain and cosmetic advantages are some of the advantages attributed to endovascular therapy compared to hysterectomy and bilateral salpingo-oophorectomy.
We should also mention the shorter time elapsed between diagnosis and treatment. As a matter of fact, the TE is often performed immediately after diagnosis through GIDP.
A prospective Italian study of 23 women confirmed the complete remission of pelvic pain and lack of pelvic varicocele in all the patients for at least 12 months after bilateral ligation of the origin of both ovarian veins performed through laparoscopy (65). Still, the number of patients treated is very small and more studies are needed before these conclusions can be confirmed. All the studies that confirmed the utility of vascular ligations through laparoscopy or laparotomy are observational studies or case reports and lack consistency. For all this, the value of these therapies has not been included conclusively in the clinical practice protocols (66).
These are reports on ovarian vein ligation, but no controlled or randomized clinical trials have analyzed the long-term safety and efficacy profile of the procedures described. The meta-analyses conducted give laparoscopic or laparotomic surgical treatments for the management of PCS a Level of Evidence 2B. Actually, these treatments are only indicated when minimally invasive techniques are unavailable or prove ineffective (67).
The limitations of laparoscopy for the diagnosis and treatment of pelvic vascular conditions are multiple and non-negligible. The increase of intra-abdominal pressure due to the pneumoperitoneum created and the Trendelemburg position both mask the presence of diseased dilated veins. That is why diagnostic laparoscopy is often normal in 80% to 90% of the cases of PCS (68).
Hysterectomy with or without bilateral oophorectomy as the therapeutic option for the management of PCS has fallen into disuseand no recent reports can be found in the medical literature available today.
We need to identify whether venous congestive clinical signs are pure or associated with compression signs like those of the PCS. To this point, multiple therapeutic options are available. However, with the arrival of new venous dedicated stents and their corresponding studies, the endovascular approach to stent implantation into the left renal vein associated with gonadal embolization should be the first-line therapy. Results are satisfactory too.
Hybrid techniques of selective ligation of pelvic leak points associated with ultrasound-guided foam sclerotherapy follow the CHIVA strategy. Extensive knowledge of these technique is required. The limitations reported are the lack of selective treatments for intrapelvic incompetent vessels and the complications associated with minimally invasive approaches.
The TE endovascular procedures performed using the sandwich technique (foam-coils-foam) to treat pelvic congestion, gonadal and hypogastric vein incompetence are minimally invasive, reproducible,outpatient with low morbidity, and highly effective (in some series efficacy is up to 95%).
The management of PCS requires a heart team, and both the diagnostic and therapeutic options should be individualized for every patient. Also, the treating team should be experiencedand savvy inall the interventional techniques available. Conventional surgery with gonadal ligation andannex-hysterectomy do not solve the leak points or the collaterality created. Instead, theyincreases morbidity and varicose vein relapse.
The endovascular techniques used to perform TE with or without a PTCA showed a high level of efficacy and effectiveness and a low rate of relapse. Still, more studies and follow-up periods are needed on this regard. Minimally invasive procedures with low morbidity should always be prioritized over the open conventional ones because of their satisfactory short- and long-term results.
4.6.6 Recommendations
|
1 |
We recommend the endovascular option for the management of congestive or compression pure or associated PCS. (Level of Evidence A, Recommendation Class Ib). |
|
2 |
Both conservative and non-conservative surgery of vascular ligation and are ill-advised due to their high morbidity and relapse. (Level of Evidence A, Recommendation Class IIa). |
|
3 |
Non-conservative surgery like hysterectomy and salpingo-oophorectomy is indicated in patients outside their reproductive lifewithPCS related tumors. (Level of Evidence B, Recommendation Class I). |
|
4 |
To this day, the endovascular procedure or open surgery is under discussion for the management of PCS. We recommend endovascular procedures due to their minimal morbidity and high level of efficacy. (Level of Evidence B, Recommendation Class IIa). |
4.6.7 References
- Reginald PW, Adams J, Franks S, Wadsworth J, Beard RW. Medroxyprogesterone acetate in the treatment of pelvic pain due to venous congestion. Br J Obstet Gynaecol 1989; 96:1148.
- Díaz- Reyes CG. Várices pélvicas y síndrome de congestión pélvica en la mujer. Rev CES Med 2012; 26(1): 57-69.
- Rundqvist E, Sandholm LE, Larsson G. Treatment of pelvic varicosities causing lower abdominal pain with extraperitoneal resection of the left ovarian vein. Ann Chir Gynaecol 1984;73:339-41.
- Beard RW, Kennedy RG, Gangar KF, Stones RW, Rogers V, Reginald PW, et al. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. Br J Obstet Gynaecol 1991;98:988-92.
- Lechter A. Pelvic várices: treatment. J Cardiovasc Surg 1985; 26:111.
- Mathis B, Miller J, Mathew L, Paluzzi M. Pelvic congestion syndrome: A new approach to unusual problem. Am Surg 1995; 61:1016-18.
- Gómez G. Varicocelectomía por laparoscopia: Descripción de una nueva técnica y su acción sobre el dolor pélvico. Med Reprod 1998; 1:14-17.
- Navarro H, Escobar MF, Fonseca J. Síndrome de congestión pélvica: Utilidad del tratamiento laparoscópico. Rev Colomb Obstet Ginecol 2005; 56: 11-17.
- Gargiulo T, Mais V, Brokaj L, Cossu E, Melis GB. Bilateral laparoscopic transperitoneal ligation of ovarian veins for treatment of pelvic congestion syndrome. J Am Assoc Gynecol Laparosc 2003;10:501-4.
- O’Brien and Gillespie. Treatment of pelvic congestion. Vasc Surg: Venous and Lym Dis 2015;3:96-106.
- Meissner MH, Gibson K. Clinical outcome after treatment of pelvic congestion syndrome: Sense and nonsense. Phlebology. 2015;30(suppl 1):73-80.
- Phillips D, Deipolyi A, Hesketh R y col. Pelvic Congestion Syndrome: Etiology of Pain, Diagnosis, and Clinical Management. J Vasc Interv Radiol 2014;25:725–733.
- Nicholson T, Basile A. Pelvic congestion syndrome, who should we treat and how? Tech Vasc Interv Radiol. 2006;9(1):19-23.
- Gutvirtz G, Imterat M, Weintraub A. Pelvic congestion syndrome: a current review. Pelviperineology 2018; 37: 14-16.
- Chapron Ch, Querleu D, Bruhat MA, Madelant P, Fernández H, Pierre F. Surgical complications of diagnostic and operative gynaecological laparos- copy; a series of 29 966 cases. Hum Reprod 1998; 8: 867-72.
- Saidi MH, Vancaillie TG, White AJ, Sadler RK, Akright BD, Farhart S. Complications of major operative laparoscopy. a review of 452 Cases. J Reprod Med 1996; 41: 471-76.
- Lehmann-Willenbrock E, Riedel HH, Mecke H, Semm K. Pelviscopy/Laparoscopy and its complications in Germany, 1949-1988. J Reprod Med 1992; 37: 671-7.
- Lynn S, Katz A, Ross P. Aortic perforation sustained at laparoscopy. J Reprod Med 1982; 27: 217-9.
- Nezhat F, Brill A, Nezhat C, Nezhat C. Traumatic hypogastric artery bleeding controlled with bipo- lar diseccation during operative laparoscopy. J Am Assoc Gynecol Laparosc 1994; 1: 171-3.
- Shifren J, Adlestein L, Finkler N. Asystolic car- diac arrest. A rare complication of laparoscopy. Obstet Gynecol 1992; 79: 840-1.
- Hershlag A, Loy R, Lavy G, DeCherney A. Femoral neuropathy after laparoscopy. A case report. J Reprod Med 1990; 35: 575-6.
- Soderstrom R. Bowel injury litigation after laparoscopy. J Am Assoc Gynecol Laparosc 1993; 1: 74-7.
- Saidi M, Sadler R, Vancaillie T, Akright B, Farhart S, White A. Diagnosis and Management of Serious Urinary Complications After Major Operative Laparoscopy. Obstet Gynecol 1996; 87: 272-6.
- Monedero, J. Pelvic congestion syndrome can be treated operatively with good long-term results. Phlebology, 27 Suppl, 1,65-73. 2012.
- Asciutto, G. & Asciutto, K. C. Pelvic Venous Incompetence: Reflux Patterns and Treatment Results. Eur J Vasc Endovasc Surg, 38, 381-386. 2009.
- Hartmann KE, Ma C, Lamvu GM, Langenberg PW, Steege JF, Kjerulff KH. Quality of life and sexual function after hysterectomy in women with preoperative pain and depression. Obstet Gynecol 2004;104:701–9.
- Nieber TE, Hendriks JC, Bongers MY, Vierhout ME, Kluivers KB. Quality of life after laparoscopic and abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol 2012;119:85–91.
- Kuppermann M, Learman LA, Schembri M, Gregorich SE, Jackson RA, Jacoby A, et al. Contributions of hysterectomy and uterus-preserving surgery to health-related quality of life. Obstet Gynecol 2013;122:15–25.
- Chung MH, Huh CY. Comparison of treatments for pelvic congestion syndrome. Tohoku J Exp Med. 2003 Nov;201(3):131-8.
- Beard RW, Kennedy RG, Gangar KF, et al. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. Br J Obstet Gynaecol 1991; 98:988–992.
- Carter J. Surgical treatment for chronic pelvic pain. J Soc Laparoendosc Surg 1998; 2:129–139.
- Frank F. Tu, MD, MPH,David Hahn, MD, and John F. Steege, MD. Pelvic Congestion Syndrome-Associated Pelvic Pain: A Systematic Review of Diagnosis and Management. Volume 65, Number 5 Obstetrical And Gynecological Survey.
- Lee NC, Dicker RC, Rubin GL, Ory HW. Confirmation of the preoperative diagnoses for hysterectomy. Am J Obstet Gynecol 1984;150:283–7.
- Beard RW, Kennedy RG, Gangar KF y col. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. Br J Obstet Gynaecol 1991;98(10):988–992.
- Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol 2007;110:1091–5.
- E. Recari, L.C. Oroz, J.A. Lara. Complications of gynaecological surgery. Anales Sis San Navarra vol.32 supl.1 Pamplona 2009.
- Leal Monedero J. Indicaciones Y Tratamiento Del Síndrome De Congestión Pélvica. Flebología Y Linfología - Lecturas Vasculares / Año 5 Nº 14 / Mayo-Agosto 2010.
- Alvarado L et al. Is the Nutcracker Syndrome the most important actor in the presence and presentation of the pelvic congestion syndrome? Journal of Vascular Surgery, Vol. 68, Issue 5, e125–e126. November 2018
- Raju S. Treatment of iliac-caval outflow obstruction. Semin Vasc Surg 2015;28:47-53.
- Murphy EH, Davis CM, Journeycake JM, DeMuth RP, Arko FR. Symptomatic ileofemoral DVT after onset of oral contra- ceptive use in women with previously undiagnosed May- Thurner syndrome. J Vasc Surg 2009;49:697-703.
- Jones WM, Taylor I, Stoddard CJ. Common iliac vein compression syndrome occurring in siblings. Br J Surg 1973;60:663-4.
- La Hei ER, Appleberg M, Roche J. Surgical thrombectomy and stent placement for iliac compression syndrome. Australas Radiol 1997;41:243-6.
- DeStephano CC, Werner EF, Holly BP, Lessne ML. Diagnosis and management of iliac vein thrombosis in pregnancy resulting from May-Thurner Syndrome. J Perinatol 2014;34:566-8.
- Nitin Garg, MBBS, MPH,a Peter Gloviczki, MD. Factors affecting outcome of open and hybrid reconstructions for nonmalignant obstruction of iliofemoral veins and inferior vena cava. JOURNAL OF VASCULAR SURGERY. Volume 53, Number 2. February 2011.
- Razavi M, Jaff M, Miller L. Safety and effectiveness of Stent placement of Iliofemoral Venous outflow obstruction systematic review and Meta-analysis. Circ Cardiovasc interv. 2015 Oct;8(10):e002772.
- Young Erben, Peter Gloviczki et al. Treatment of Nutcracker syndrome with open and endovascular interventions. Journal of Vascular Surgery: Venous and Lymphatic Disorders, Vol. 3, Issue 4, p389–396. June 29, 2015.
- Monedero JL, Zubicoa Ezpeleta S, Nl M. Khilnani. Treatment options for pelvic congestion syndrome Phlebolymphology - vol 23. No. 3. 2016.
- Wang L, Yi L, Yang L, Liu Z, Rao J, Liu L, et al. Diagnosis and surgical treatment of nutcracker syndrome: a single-center experience. Urology 2009;73:871-6.
- Pastershank SP. Left renal vein obstruction by a superior mesenteric artery. J. Can. Assoc. Radiol. 25, 52–54 (1974).
- Velasquez et al. A systematic review on management of nutcracker syndrome. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 273 Volume 6, Number 2. March 2018.
- Gong XY, Zheng W, Du H, Lei Y, Xue YH, Xue CH, et al. Treatment of nutcracker syndrome with spermatic vein ligation and iliac vein anastomosis: case report of three cases. Asian Pac J Trop Med 2012;5:923-4.
- A.H. Scultetus, J.L. Villavicencio, D.L. Gillespie. The nutcracker syndrome: Its role in the pelvic venous disorders. J Vasc Surg, 34 (2001), pp. 812-819.
- Benrashid E. Nutcracker Syndrome. Rutherford’s Vascular Surgery and Endovascular Therapy, E-Book. Chapter 165.
- Ali-El-Dein B, Osman Y, Shebab El-Din AB et al. Anterior and posterior nutcracker syndrome: a report on 11 cases. Transplant. Proc. 35, 851–853 (2003).
- Stewart BH, Reiman G. Left renal venous hypertension ‘nutcracker’ syndrome. Managed by direct renocaval reimplantation. Urology 20, 365–369 (1982).
- Reed NR, Kalra M, Bower TC et al. Left renal vein transposition for nutcracker syndrome. J. Vasc. Surg. 49, 386–393 (2009).
- Chau et al. Robotic-assisted left renal vein transposition as a novel surgical technique for the treatment of renal nutcracker syndrome. Journal of Vascular Surgery Cases and Innovative Techniques Volume 4, Number 1. March 2018.
- Wang SZ, Zhang WX, Meng QJ, Zhang XP, Wei JX, Qiao BP. Laparoscopic extravascular stent placement for nutcracker syndrome: a report of 13 cases. J Endourol 2015;29:1025-9.
- Zhang Q, Zhang Y, Lou S, Liu F, Ye Z, Zhang D. Laparoscopic extravascular renal vein stent placement for nutcracker syndrome. J Endourol 2010;24:1631-5.
- Neste MG, Narasimham DL, Belcher KK. Endovascular stent placement as a treatment for renal venous hypertension. J. Vasc. Interv. Radiol. 7, 859–861 (1996).
- WuZ,ZhengX,HeY,FangX,LiD,TianL,etal.Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord 2016;4:193-9.
- Chen S, Zhang H, Shi H, Tian L, Jin W, Li M. Endovascular stenting for treatment of nutcracker syndrome: report of 61 cases with long-term followup. J Urol 2011;186:570-5.
- Chen W, Chu J, Yang J et al. Endovascular stent placement for the treatment of nutcracker phenomenon in three pediatric patients. JVIR 16, 1529–1533 (2005).
- Leal Monedero J, Zubicoa Ezpeleta S, Sánchez Galán A, Perrin M, Management Of Left Renal Vein Compression In Patients Presenting Left Gonadal Vein Reflux. Controversies and Updates in Vascular Surgery. January 2018. Paris.
- Borghi, C y Dell Atti, L. Pelvic congestion syndrome: the current state of the literature. Arch Gynecol Obstet (2016) 293: 291-301.
- Gloviczki, P; Comerota, A; Dalsing M y col. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the american Venous Forum. J Vasc Surg (2011) 53(5 SUPPL.): 2S-48S.
- Freedman, J; Ganeshan, A y Crowe P. Pelvic congestion syndrome: the role of interventional radiology in treatment of chronic pain. Postgrad Med J (2010); 86:704-710.
- Jane P. Daniels, PhD, Rita Champaneria. Et al. Effectiveness of Embolization or Sclerotherapy of Pelvic Veins for Reducing Chronic Pelvic Pain: A Systematic Review. J Vasc Interv Radiol 2016; 27:1478–1486.
4.7 Additional extrapelvic management
Authors: Oscar Gural Romero, Juan Nigro, Miguel Amore
4.7.1 Introduction
PCS can express itself exclusively in the LL through pelvic leaks characterized by varicose veins of atypical location and symptoms such as pain and heaviness. As evidence of a more extensive and severe damage, both the pelvis and the LL can be clinically compromised in the PCS. The vascular surgeon or the phlebologists often find the first signs of suspected PCS after surgery in the varicose vein recurrence of the LL.
This chapter describes the importance of bearing in mind that PVR requires the precise assessment of its spread into the LL. In the management of postoperative varicose vein recurrence, a detailed assessment is required to evaluate the presence of PFL due to possible PCS. Once the pelvic varicose veins have been confirmed as the origin of the LL venous incompetence, the sequential TE management of these varicose veins and PFL followed by surgery of the LL varicose veins can be considered.
In the post-TE management of PCS with atypical varicose veins in the LL, some patients’ extrapelvic varicose signs relapse with or without symptoms. In these cases, it is necessary to proceed with the closure of the PFL through imaging-guided procedures to prevent new LL varicose recurrences. Below is a description of the level of significance of the procedures used to control LL venous incompetence of pelvic origin. The results of these procedures are still being studied.
4.7.2 Comprehensive diagnostic evaluation
of pelvic venous incompetence
The incidence rate of the association between pelvic, vulvar, and perineal varicose veins and the LL is somewhere around 80% (1-4). Pelvic varicose veins were found in simple diagnostic imaging modalities in 30 out of 100 women with CPP studied using TVCDE. Twenty-one of these women (70%) had some sort of LL venous incompetence (5).
Other authors have established that between 15% and 20% of all LL varicose veins and 20% to 80% of all postoperative varicose recurrences have a partial or complete pelvic origin (6,7).
PCS represents 35% of the cases of vulvovaginal varicose veins, and 90% of the cases of LL varicose veins (8,9).
Gonadal vein incompetence can cause the recanalization of round ligament veins and hypogastric vein branches like the internal pudendal, obturator, and inferior gluteal vein, which can create leaks into the LL and the vulvar region (Figures 21, and 22).
These abdominopelvic connections with the LL can have four main pathways:
1. Saphenofemoral junction reflux.
2. Reflux into the great and small saphenous veins.
3. Reflux parallel to the saphenous axis in the thigh medial compartment.
4. Sciatic reflux into the thigh and gluteus posterior sectors (4).
It is important to correlate the semiological examination with the performance of superficial and deep venous DCE of the LL, abdominopelvic, and transvaginal regions. In cases of suspected compression syndrome, incompetence or pelvic reflux it is complemented with a PTCA, a CAT scan or an MRI. The endovascular therapy of TE and/or PTCA should be selected based on the pathophysiology diagnosed (10) (Figure 23).
The TE of PCS often targets extrapelvic atypical veins partially. These remnants of perineal, vulvoperineal or gluteal varicose veins perpetuate the LL venous incompetence.
Therefore, if overt clinically significant vulvoperineal varicose veins are confirmed on the CDE during the post-TE management at the 3- and 12-month follow-up, it is advisable to extend the TE as distal as possible from the PFL found in the clinical relapse and on the CDE.
There are times that it will be necessary to complete the reassessment of the cases through a CAT scan, an MRI or a varicography.
The recurrent varicose vein study protocol includes the assessment of:
• The TE procedure:
o Veins treated, methods used, and results.
o Possible presence of compression inducedcondition.
o Possible use of stent.
• Simple inspection and using augmented reality (Vein Viewer): perineal, vulvar, abdominal, gluteal, and LL.
• CDE:
o LL, abdomen, and pelvis, TV.
• Abdominopelvic images:
o CAT scan, MRI, PTCA, varicography.
4.7.2.1 Clinical-semiological examination
Figure 24 shows the 6 PFL points in the front, posterior, and gynecological or diamond positions. Their assessment should include the search for the origin of perineal varicose veins.
The inguinal point shows round ligament and gonadal vein incompetence; the clitoridian and perineal points, internal pudendal vein compromise; the obturator point, the damaged obturator vein, while the superior and inferior gluteal points derive from the gluteal veins and can be connected withthe thigh posterior side sciatic veins (11).
4.7.2.2 Semiological examination under augmented reality
Vein Viewer is an imaging modalitythat shows veins down to 1 cm deep and identifies reticular and truncular vessels that cannot be seen directly. In thin patients, it can identify perivulvar, clitoridean, and truncular vessels originated at gluteal, pudendal and obturator leak points.
It is used during vascular access, leak point mapping, and as an imaging modality to assist with guiding sclerotherapy andthrombectomy (Figure 25).
4.7.2.3 Color-coded Doppler echocardiography
The abdominopelvic CDE performed in semi-sitting position raised at a 45-degree angle and in the decubitus dorsal position allows us to see gravitational flow and assess iliac vein refluxes and their pudendal and obturator territories in already embolized patients.
The main obstacle to these examinations is obesity, which is often overcome when the TVCDE is used to assess low pelvic refluxes. Also, it can identify reflux coming from the ovarian veins by finding its origin. Similarly, given the nearness of the transducer to the internal iliac tributary veins, it can detect refluxes coming from these branches (Figure 26). Finally, the TVCDE is also used to assist with guiding ultrasound-guided percutaneous sclerotherapy.
The venous CDE of the LL can identify 4 different patterns: 1, normal pattern; 2, reflux pattern, 3; compression pattern or 4, mixed pattern. It is also important to define the correlation between incompetent veins and saphenofemoral and saphenopopliteal arches, great and small saphenous trunk segments, and leak and reentry perforator veins. It also shows if the incompetent vessel found has intra- or extrapelvic origin as well as its anatomical correlations (12).
4.7.2.4 Computed axial tomography and magnetic
resonance imaging
The multislice CAT scan with 3D reconstruction or the MRI with venous time allow us to confirm the presence of associated compression patterns, assess the possible bypass pathways, pelvic varicocele, and even associated DVT (Figure 27).
4.7.2.5 Gonadal and iliac dynamic phlebography of pelvic floor leak
The ascending phlebographyand the gonadal and iliac dynamic phlebography (GIDP) (or descending) in apnea and with the Valsalva maneuver identify various leak and reflux points, and with the CDE phlebologic mapping as the reference, the intrapelvic TE can be planned followed by an additional extrapelvic TE (Figures 28, 29 and 30).
4.7.2.6 Varicography
It shows the venous diameters and the hemodynamics of the circulatory system and spread to be able to plan the volume and concentration of embolization material (Figure 31).
Traditionally, in the definition of postoperative recurrences of LL venous incompetence, there has been a lack of uniformity in the criteria to be met in the methods used for outcome assessment and follow-up duration. The frequency of this postoperative recurrence of LL varicose veins due to venous incompetence of pelvic origin is between 17% and 34%, Also, medial vein reflux into the saphenofemoral junction is predominant here (68%) and, to a lesser extent, from the gluteal veins (32%) (13-15).
4.7.3 Therapeutic arrangement of lower limb
venous incompetence originated from
pelvic varicose veins reflux
Once the diagnosis of pelvic venous incompetence has been established as the cause of LL varicose veins, treatment should be directed to the early control of pelvic venous hypertension and its PFL points through catheterization and TE. Similarly, if pelvic varicose veins are due to iliac venous or left renal vein compression (MTS or NCS), endovascular correction with PTCA or renal venous surgery should be prior to the resolution of the LL varicose veins (16-18).
It has been confirmed that the TE of PVR improves the clinical signs of LL varicose veins (19) and even achieves full clinical resolution (4).
4.7.3.1 Common pelvic floor leak points
1. Inferior gluteal or sciatic vein incompetence with inferior gluteal leak point, sciatic veins in association with varicosities at external or posterior thigh-calf level with reentry into small saphenous vein (Figure 32).
2. Saphenofemoral arch relapse with a history of major saphenectomy due to obturator or internal pudendal vein incompetence and vessel neo-formation in association with the thigh anterior saphenous vein or varicosities in the saphenectomy bed territory (Figure 33).
3. Isolated varicose veins in the thigh internal, external, and posterior side or in the inguinal or gluteal region in association with right gluteal leaks (Figure 34).
4. Internal pudendal leak point in association with incompetent great, small, and accessory saphenous trunks (Figure 35).
4.7.3.2 Treatment techniques of pelvic floor leak points
Percutaneous extrapelvic embolization
It allows us to complete the treatment of all the segments compromised with excellent response. Also, it allows us to treat the saphenous, perforator or communicating trunks at LL level in the same surgical act.
During the first control, mapping of the PFL points is performed with the perineal or gluteal vulvar varicose epifascial vessels and reflow direction by combining CDE operating in the frequency range of 2 to 18 megahertz and Vein Viewer. Afterwards, the ultrasound-guided sclerotherapy is completed by viewing the vein and the trajectory of the foam across the target vein.
At the operator’s discretion, the Vein Viewer is used alone or in combination with a high-resolution ultrasound for sclerotherapy guidance purposes.
Direct Vision Sclerotherapy using Vein Viewer
Under augmented reality guidance the dominant vessel can be identified and the direction of flow can be established. The vessel sclerotherapy is performed using the foam technique, which indirectly allows us to treat the associated leak point (Figure 36).
Técnica de esclerosis con foam en forma directa ecoasistida y Vein Viewer (Figura 37)
Foam sclerotherapy is preferably performed with polidocanol. Its concentration varies depending on the target vessel diameter (vulvar varicose veins, polidocanol at 0.5%; perivulvar or perineal epifascial varicose veins, polidocanol at 1%; pudendal, inguinal, obturator, and gluteal leak points, polidocanol at 2%). Proxima-to-distal sclerotherapy should be performed, and the Valsalva maneuver should be requested to facilitate the puncture and increase the target vessel diameter. The maximum amount of infusion should be 10 cm of foam and 3 cm3 of sclerosing solution followed by selective compression for 6 hours.
Follow-up is conducted once a year through CDE at the 1-, 3-, 6-, and 12-month mark. Thrombectomy is performed under augmented reality of significantly thombosed segments starting 10 days after the sclerotherapy, reducing thrombophlebitis (Figure 38).
Technique of Conventional Ultrasound-guided Sclerotherapy (Figures 39, and 40).
This technique is traditionally used for all ultrasound-guided sclerotherapieswith high-frequency transducers. This technique not only works on skin-to-vessel distances, but also helps measurethe diameters and degrees of reflux correctly.
Section and selective ligation of leak points associated with intra- and extrapelvic sclerosis (20) (Figura 41)
There is a therapeutic current of hybrid open surgery procedures with leak point ligation associated with foam sclerotherapy described by Franceschi et al. The intrapelvic endovascular therapy is not performed here. The authors claim that this double approach the condition can be treated both at abdominal and pelvic level. The setbacks of this technique are the neurological lesion complications, chronic pain, cosmetic complications, and infections reported. Also, its capacity to occlude intrapelvic vessels like the gonadal ones or the hypogastric vein great tributary vessels has been put into question.
4.7.4 Postoperative management
It is clinically performed through TVCDE at the 1-, 3-, 6-, and 12-month mark (Figures 42, and 43).
The reasons why postoperative TE recurrences occur are due to different causes such as:
• Assessment errors of venous drainages of pelvic cells on the GIDP.
• Misdiagnosed venous anatomical variables.
• Presence of superficial and/or deep essential or post-thrombotic venous incompetence.
• Angiodysplasias.
• Technical errors or technical deficiencies:
- Insufficient or incomplete treatment of vascular axes.
- Lack of treatment of associated compression syndromes: NCS and MTS.
- Use of the embolization technique with coils only.
- Selection of inadequate materials.
• Post-embolization pregnancies.
We recommend the combined intra- and extrapelvic therapy to treat reflux because with the former alone, the painful symptoms of a large percentage of patients don’t go away or the signs of congestion or the pain from the varicose veins, especially, at genital level.
Knowing that with the TE with coils and foam it is not always possible to treat the most distal segments like those at the gluteal or pudendal regions, additional extrapelvic embolization is needed to complete the treatment of all the compromised segments with an excellent response. Also, the saphenous, perforator or communicating trunks present in the LL should be treated as well.
4.7.5 Treatment of intrafascial and epifascial vessels
Different techniques can be considered to treat saphenous or accessory trunks and perforator leak vessels that can be categorized into conventional or minimally invasive techniques.
4.7.5.1 Conventional surgery
Conventional major or minor saphenectomy (arch ligation with saphenous veinphleboextraction). Phleboextraction technique with invagination
Resection of varicose segments using a stepped approach. Müller’s Technique.
Perforator vein surgery. Section and ligation, Cigorraga valve repair or Lacour valve repair, Lintonsugery, Cockett surgery or Linton Cockett surgery, etc.
Conventional surgeries like saphenectomy have a series or requirements such as (24):
• Spinal or general anesthesia.
• Need for hospitalization, even though it is an outpatient procedure.
• Traumatic technique. Walking limitations.
• Impossibility to treat bilateral multiple saphenous trunks.
The set backs of conventional surgery are its higher rate of complications like infections (2% to 6%), hematomas, neurological lesions (14% to 30%), deep venous thrombosis (0.4% to 5.3%), and pulmonary thromboembolism (0% to 0.5%). The rate of varicose recurrence at the 5-year follow-up is 20% to 50% due to the neovascularization of the arch (25-27).
4.7.5.2 Minimally invasive procedures
Non-endovascular
• SEPS (subfascial endoscopic perforating surgery). It consists of the endoscopic subfascial ligation of perforator veins.
Endovascular
• Thermal ablation with laser, radiofrequency or water vapor.
• Ultrasound-guided sclerotherapy. Foam: saphenous, perforator and communicating trunks.
• Mechanochemical ablation (MOCA): saphenous trunks.
• Cyanoacrylate: saphenous and perforator trunks.
Conventional techniques were the standard of use until the arrival of CDE, which allowed us to perform minimally invasive procedures with less morbidity, shorter down times (both on the social and working level), a high efficacy level like sclerotherapy, thermal ablations, mechanochemical ablations, and cyanoacrylate (Level of Evidence 1A) (21-23).
Thermal, mechanochemical, and cyanoacrylate-based endovascular procedures all share common characteristics:
• No saphenofemoral or saphenopopliteal arch ligation.
• Local anesthesia with support or neuroleptoanalgesia.
• Treatment of the arch in a selective endovascular fashion.
• Use of safety margins.
• Ultrasound-guided puncture and procedures.
• Use of tumescent anesthesia in thermal ablations.
• Totally performed on an outpatient basis; patients remain in observation status for 1.5 to 2 hours.
The most common complications are hematomas (30%-60%), pigmentation (1%-10%), thrombosis in the saphenous proximal remnant or spread towards the femoral vein (< 3%), thrombophlebitis (5.6%; range: 4.2%-7%), skin burns (< 1%), paresthesia (3.8%; range: 2.4%-4.5%), and infection (0.5%; range: 0.3%-1.3%). All of them are less important or significant complications compared to those of conventional procedures (24).
Today, the treatment of choice is a hybrid procedure that includes different techniques (Figure 44).
In this case, in view of the presence of an incompetent bilateral great saphenous vein due to previous surgery, varicose relapse by the thigh anterior accessory segment originated at the obturator and bilateral internal pudendal leak point in association with the saphenofemoral arch, an early intrapelvic TE of leak points with the foam-coils-foam technique was performed. Afterwards, subsequent different therapeutic options were considered (Figure 45):
• Laser or endoluminal radiofrequency of the thigh anterior accessory vein intrafascial trajectory followed by epifascial varicose segments resection using a stepped approach.
• Laser or endoluminal radiofrequency of the thigh anterior accessory vein intrafascial trajectory with foam injection into the epifascial varicose segments.
• Ultrasound-guided foam of the thigh anterior accessory vein and epifascial vessels.
• Reassessment of the arch with ligation of the relapsed arch followed by epifascial varicose segment resection using a stepped approach.
• Augmented reality-assisted sclerotherapy of epifascial vessels.
Figure 46 shows the inferior gluteal leak point in continuity with sciatic veins and evidence of epifascial varicose veins on the thigh and leg posterior side. Intrapelvic TE treatment is advised to later consider other therapeutic options for the epifascial vessels:
• Ultrasound-guided foam sclerotherapy.
• Subfascial vessel sclerotherapy associated with stepped resection.
• Subfascial vessel sclerotherapy associated with laser or radiofrequency of epifascial vessels.
• Laser or radiofrequency of intrafascial vessels associated with stepped resection or foam injection of epifascial segments (not recommended due to the risk of tributary sciatic nerve thermal lesion).
4.7.6 Analysis and considerations
Few international studies have been published on the post-embolization management of remnant or recurring extrapelvic veins. However, therapies with liquid sclerosing agents have been performed for quite a few decades now. Still, few cases in the medical literature available describe the use of sclerosing foam as a suitable therapy.
Anatomically speaking, the size of pelvic floor veins or their extrapelvic projections is small (0.1 mm to 1 mm in diameter) or medium (1 mm to 5 mm). The smallest ones have a tunica intima composed of endothelial cells surrounded by connective tissue whose volume increases parallel to the volume of blood flow. Their tunica media is very thin with circular layers of highly loose smooth muscle cells. Finally, their tunica adventitia is made up of loose connective tissue, which is why the action of the sclerosing detergent and its physical combination with foam cause instantaneous spasm/stenosis and quick fibrosis.
This situation validates the assessment of the concentrations and volumes that should be used, which will depend on the type of vein, diameter, and spread towards its intrapelvic drainages. Overall, post-TE recurrences are due to technical defects and the most common thing of all is the permanence of venous remnants resulting in the coexistence of essential and/or chronic LL venous incompetence. These situations were classified by Franceschi et al. as type 4 venous shunts. Remnant veins are the product of great extrapelvic dilatations due to severe venous hypertension and reflux into the distal portion which, due to its spread into the LL, could not be treated completely with endovascular therapy. Primary PCS or due to compression or post-thrombotic syndromes can occur in LL as atypical varicose veins or through PFL. Under certain circumstances it is associated with saphenous trunk and perforator vein incompetence. It is necessary to establish a correct correlation between the clinical examination and any additional tests. Here the CDE plays an important role because it can identify different ultrasound patterns, leak points, and associations with the superficial, deep and the perforator vein system.
The GIDP is not only diagnostic but also allows us to treat pelvic refluxes or associated signs like the NCS and the MTS. Therapy should be combined and individualized in each case: first, intrapelvic resolution followed by the extrapelvic resolution of leak points and the resolution of vertical or horizontal venous refluxes at LL level.
In our own experience with extrapelvic embolization added to intrapelvic TE, the results were therapeutically good. However, the follow-up on these procedures is still too scarce and short to draw any possible, safe, and definitive conclusions. The international medical literature on these cases of extrapelvic leaks is also scarce. However, the results of pelvic TE have are highly effective as well as the management of remnant extrapelvic veins with these minimally invasive percutaneous techniques. In conclusion, the correct and complete TE of intrapelvic veins reflux is useful as coadjuvant therapy of LL varicose veins due to hypertensive leaks with extrapelvic signs. That is why the therapeutic priority in symptomatic patients with PCS and LL varicose veins is pelvic TE.
In patients with etiology of LL venous incompetence and simultaneously PCS, a therapeutic strategy should be established on a global level with accurate action times for result optimization and preventing relapse and recurrence. Multidisciplinary management is advisable to optimize diagnosis, the intra- and extrapelvic therapy of PCS, as well as for the proper follow-up of these complex patients.
4.7.7 Recommendations
|
1 |
Start by solving intrapelvic venous congestion via an endovascular approach and then proceed to complete the extrapelvic component. (Level of Evidence B, Recommendation Class I). |
|
2 |
Additional treatment of extrapelvic leaks with sclerotherapy in all its modalities using augmented reality, Vein Viewer, and high-resolution CDE. (Level of Evidence B, Recommendation Class I). |
|
3 |
Additional resolution of superficial and perforator LL venous incompetence after intrapelvic embolization. Minimally invasive techniques like thermal ablation with laser or radiofrequency and ultrasound-guided sclerotherapy are advised over conventional surgical techniques due to their high level of efficacy, minimal relapse rate, and shorter down times (both on the social and working level). (Level of Evidence A, Recommendation Class I). |
4.7.8 References
- Hobbs JT. The pelvic congestion syndrome. Br J Hosp Med 1990,43:200-6.
- Desimpelaere JH, Seynaeve PC, Hagers YM, Appel BJ, Mortelmans L.L. Pelvic congestion syndrome: demonstration and diagnosis by helical CT. Abdom Imaging 1999,24:100-2..
- Gültaslı NZ, Kurt A, Ipek A, et al. The relation between pelvic varicose veins, chronic pelvic pain and lower extremity venous insufficiency in women. Diagn Interv Radiol 2006;12:34-8.
- Oliveira FA, De Sousa Amorelli CE, Lemos Campedelli F, at al. Treatment of pelvic congestion associated with varicose veins of the lower limbs: report of a small number of cases. J Vasc Bras 2012;11(1):62-6.
- Bora A, Avcu S, Arslan H, Adali E, Bulut MD. The relation between pelvic varicose veins and lower extremi- ty venous insufficiency in women with chronic pelvic pain. JBR–BTR, 2012;95:215-21.
- Marsh P, Holdsock J, Harrison C, Smith C, Price BA, Whiteley MS. Pelvic vein reflux in female patients with varicose veins: comparison of incidence between a specialist private vein clinic and the vascular department of a National Health Service District General Hospital. Phlebology 2009;24(3):108-13.
- Whiteley AM, Taylor DC, Whiteley MS. Pelvic venous reflux is a major contributory cause of recurrent varicose veins in more than a quarter of women. J Vasc Surg 2013;1:100-1.
- Holdstock JM, Dos Santos SJ, Harrison CC, Price BA, Whiteley MS. Haemorrhoids are associated with internal iliac vein reflux in up to one-third of women presenting with varicose veins associated with pelvic vein reflux. Phlebology 2015;30:133-9.
- Lemasle P, Greiner M. Duplex ultrasound investigation in pelvic congestion syndrome: technique and results. Phlebolymphology 2017;24(2).
- Monedero JL, Ezpeleta SZ, Perrin M. Pelvic congestion syndrome can be treated operatively with good long-term results. Phlebology 2012;27 (suppl 1):65-73.
- P. Zamboni et al. Minimally Invasive Surgical. Treatment of Pelvic Leak Points, Saphenous Vein-Sparing Strategies in Chronic Venous Disease, https://doi.org/10.1007/978-3-319-70638-2_8
- Labropoulos N, Tiongson J, Pryor L, et al. Definition of venous reflux in lower- extremity veins. J Vasc Surg 2003;38:793-8.
- Labropoulos N. Nonsaphenous superficial vein reflux. J Vasc Surg 2001:34(5):872-7.
- Van Rij AM, Jiang P, Solomon C, et al. Recurrence after varicose vein surgery (a prospective long-term clinical study with duplex ultrasound scanning and air plethysmography). J Vasc Surg 2003;38:935-43.
- Perrin MR, Labropoulos N, Leon LR Jr. Presentation of the patient with recurrent varices after surgery (REVAS). J Vasc Surg 2006;43(2):327-34.
- Nicholson T, Basile A. Pelvic congestion syndrome, who should we treat and how? Tech Vasc Interv Radiol 2006;9:19-23.
- Sichlau MJ, Yao JS, Vogelzang RL. Transcatheter embolotherapy for the treatment of pelvic congestion syn- drome. Obstet Gynecol 1994;83:892-6.
- Hartung O. Embolization is essential in the treatment of leg varicosities due to pelvic venous insufficiency. Phlebology 2015;30(1S):81-5.
- Meneses L, Fava M, Diaz P, et al. Embolization of Incompetent Pelvic Veins for the Treatment of Recurrent Varicose Veins in Lower Limbs and Pelvic Congestion Syndrome. Cardiovasc Intervent Radiol 2013;36:128-32.
- Franceschi C, Delfrate R, Bricchi M, Quadrozzi F. Varices vulvaires après la grossesse. Doit-on lier les points de fuite? Minimally-invasive surgical procedure for pelvic leak points in women. January 2017.
- The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2011;53:2S-48S.
- Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery! and the American Venous Forum. J Vasc Surg 2014;60:3S-59S.
- Consensus. Society for vascular surgery and american venous forum guidelines on the management of venous leg ulcers: the point of view of the International Union of Phlebology. International Angiology 2015;34(3).
- Rutges P, Kitslaar P. Randomized trial of stripping versus high ligation combined with sclerotherapy in the treatment of the incompetent greater saphenous vein. Am J Surg 1993;168:311-5.
- Rasmussen LH, Bjoern L, Lawaetz M, Blemings A. Lawaetz B, Eklof B. Randomized trial comparing endovenous laser ablation of the GSV high ligation and stripping in patients with varicose veins: short term results. J Vasc Surg 2007;46:308-15.
- Huisman LC, Bruins RM, van den Berg M, Hissink RJ. Endovenous laser ablation of the small saphenous vein: prospective analysis of 150 patients, a cohort study. Eur J Vasc Endovasc Surg 2009;38(2):199-202.
- Darwood RJ, Theivacumar N, Dellagrammaticas D, Mavor AI, Gough MJ. Randomized clinical trial comparing ELVT ablationt with surgery for the treatment of primary GSV veins. Br J Surg 2008;95:294-301.
Este artículo no contiene material bibliografico
Miguel Amore
miguelangelamore@hotmail.com. 2do Jefe de Servicio de FlebologÃa y LinfologÃa, Dto. de CirugÃa Cardiovascular. Hospital Militar Central. Staff del Servicio de FlebologÃa y LinfologÃa. Fundación Favaloro.
Hernán Bertoni
hernangbertoni11@gmail.com. Radiólogo Intervencionista. Jefe de Servicio Oncointervencionismo. Instituto Roffo. Médico de Staff del Servicio de CardioangiologÃa Intervencionista. Instituto Fleni.
Pamela Causa Andrieu
pamela.causa@hospitalitaliano.org.ar. Médica Asociada. Servicio de Diagnóstico por Imágenes. Hospital Italiano de Buenos Aires, Argentina. Clinical Fellow. Department of Radiology. Memorial Sloan Kettering Cancer Center, Nueva York, EE.UU.
Luis Catalina
luismc12@gmail.com. Médico especialista en Diagnóstico por Imágenes. Universidad de Buenos Aires. Staff del Servicio de EcografÃa Vascular. Fundación Favaloro y Diagnóstico Maipú. Director Médico. Centro Diagnóstico Doppler San Miguel. Encargado de la Sección Ecodoppler Abdominal y Pélvico. Vascular Integral. Docente de SAUMB. Miembro del Grupo Iberoamericano de Estudio Pélvico.
Carolina Chacon
carolina.chacon@hospitalitaliano.org.ar. Médica de planta. Jefa de Sección de EcografÃa. Coordinadora del Ãrea de Imágenes en GinecologÃa. Servicio de Diagnóstico por Imágenes. Hospital Italiano de Buenos Aires, Argentina.
Carlos D´Alotto
carlosdalotto@gmail.com. Médico Especialista en Diagnóstico por Imágenes. Coordinador Ãrea de Doppler vascular. Diagnóstico Maipú. Buenos Aires, Argentina..
Marcelo Dándolo
mdandolo@gmail.com. Cirujano Vascular. Expresidente de la Asociación Argentina de AngiologÃa y CirugÃa Cardiovascular (AAAyCCV). Expresidente del Colegio Argentino de CirugÃa Venosa y Linfática (CACVyL). Subjefe del Servicio de FlebologÃa y LinfologÃa. Fundación Favaloro. Staff de la Unidad de CirugÃa Vascular. Hospital Perón y Sanatorio Itoiz. Miembro Titular del Colegio Argentino de Cirujanos Cardiovasculares (CACCV). Miembro Titular de la Asociación Argentina de CirugÃa. Miembro Titular del Grupo Iberoamericano de Estudio Pélvico..
Guillermo Eisele
guillermoeisele@gmail.com. Médico Especialista en Diagnóstico por Imágenes UBA y homologación España. Jefe de RadiologÃa Intervencionista. Hospital de Niños Dr. Ricardo Gutiérrez. Miembro fundador Colegio Argentino de RadiologÃa Vascular e Intervencionista.(CARVI). Docente del Curso Superior de Diagnóstico por Imágenes. Hospital de ClÃnicas, UBA.
Santiago Gil
santiago.gil@hospitalitaliano.org.ar. Médico ginecólogo especialista en fertilidad y jefe de sección de PatologÃa Pelviana Benigna. Hospital Italiano de Buenos Aires.
Néstor Giráldez
nestorgiraldez@yahoo.com.ar. Cirujano Vascular. Miembro titular del Colegio Argentino de Cirujanos Cardiovasculares de la Sociedad de FlebologÃa y LinfologÃa Bonaerense y de la Asociación Argentina de AngiologÃa y CirugÃa Vascular. Docente de la Carrera Universitaria de FlebologÃa y LinfologÃa. Universidad de Morón. Unidad de CirugÃa Vascular. Sanatorio Municipal Dr. Julio Méndez.
Sebastián Gogorza
sebastian.gogorza@hospitalitaliano.org.ar. Doctor en Medicina. Jefe honorario de GinecologÃa. Hospital Italiano. Profesor Titular de GinecologÃa. Instituto Universitario Hospital Italiano de Buenos Aires..
Oscar Gural
oagural@gmail.com. Especialista en CirugÃa Cardiovascular. Intervencionismo Venoso. Jefe de Servicio de FlebolinfologÃa. FlebologÃa Intervencionista. Fundación Favaloro. Vicepresidente del Grupo Iberoamericano de Estudio Pélvico.
Alberto Kenny
alberto.kenny85@gmail.com. Médico Especialista en Diagnóstico por Imágenes y RadiologÃa Intervencionista. Hospital Italiano, UBA. Becario Hospital Georges Pompidou, ParÃs, Francia. Staff de RadiologÃa Intervencionista de Sanatorios de Galeno Argentina y Swiss Medical Group, Buenos Aires, Argentina.
Esteban Mendaro
esteban.mendaro@hospitalitaliano.org.ar. Radiólogo Intervencionista. Director Médico de Investigaciones Vasculares. Jefe del Servicio de Hemodinamia. Sanatorio de la Providencia. Médico asociado de Diagnóstico por Imágenes. Hospital Italiano de Buenos Aires..
Noelia Napoli
maria.napoli@hospitalitaliano.org.ar. Médica Asociada. Servicio de Diagnóstico por Imágenes. Hospital Italiano de Buenos Aires, Argentina.
Juan Nigro
juananigro@hotmail.com. Cirujano Vascular Periférico. Director del Curso Superior Universitario de FlebologÃa y LinfologÃa, Universidad de Morón. Director del Curso Superior de Ecodoppler Vascular Periférico e Intervencionismos Ecodirigidos (CACCV). Vicepresidente de la Asociación Argentina de AngiologÃa y CirugÃa Cardiovascular (AAAyCCV). Jefe de Unidad de FlebologÃa y LinfologÃa, Hospital Eva Perón, provincia de Buenos Aires. Secretario general del CACCV.
Juan Paolini
juanestebanpaolini@gmail.com. Cirujano Vascular Sanatorio Dr. Julio Méndez, PoliclÃnico del Docente. Presidente Colegio Argentino de Cirujanos Cardiovasculares (CACCV). Presidente Argentine Chapter of Society of Vascular Surgery (SVS). Secretario General Asociación Latinoamericana de CirugÃa Vascular (ALCVA). Secretario General Sociedad de FlebologÃa y LinfologÃa Bonaerense (SFLB). Subdirector Curso Universitario de FlebologÃa y LinfologÃa, Universidad de Morón (UM).
Eugenio Piraino
eepiraino@hotmail.com. Médico ginecólogo especialista en Laparoscopia e Histeroscopia, Jefe de Servicio de GinecologÃa del Sanatorio San José.
Autor correspondencia
Guillermo Eisele
guillermoeisele@gmail.com. Médico Especialista en Diagnóstico por Imágenes UBA y homologación España. Jefe de RadiologÃa Intervencionista. Hospital de Niños Dr. Ricardo Gutiérrez. Miembro fundador Colegio Argentino de RadiologÃa Vascular e Intervencionista.(CARVI). Docente del Curso Superior de Diagnóstico por Imágenes. Hospital de ClÃnicas, UBA.
Correo electrónico: guillermoeisele@gmail.com
Para descargar el PDF del artículo
Intersocietal Argentine Pelvic Congestion Syndrome Consensus. Part 2
![]() Haga click aquí
Haga click aquí
Para descargar el PDF de la revista completa
Revista Argentina de CardioangiologÃa intervencionista, Volumen Año 2021 1
Revista Argentina de CardioangiologÃa intervencionista
Issue # 1 | Volumen
11 | Año 2021
Differences between randomized clin...
Alfredo E RodrÃguez
Understanding causes of death in tr...
Lucas C Godoy y cols.
Intersocietal Argentine Pelvic Cong...
Miguel Amore y cols.
Study of the utility of lorazepam i...
Ramiro Acevedo y cols.
Acute aortic regurgitation, pericar...
Carlos Fernández Pereira y cols.
Renal infarction
Natalia Mercado y cols.
With a new challenge and hopes in 2...
Diego Grinfeld
Intersocietal Argentine Pelvic Congestion Syndrome Consensus. Part 2
Autores
Miguel Amore, Hernán Bertoni, Pamela Causa Andrieu, Luis Catalina, Carolina Chacon, Carlos D´Alotto, Marcelo Dándolo, Guillermo Eisele, Santiago Gil, Néstor Giráldez, Sebastián Gogorza, Oscar Gural, Alberto Kenny, Esteban Mendaro, Noelia Napoli, Juan Nigro, Juan Paolini, Eugenio Piraino
Publicación
Revista Argentina de CardioangiologÃa intervencionista
Editor
Colegio Argentino de Cardioangiólogos Intervencionistas
Fecha de publicación
2021-03-31
Registro de propiedad intelectual
© Colegio Argentino de Cardioangiólogos Intervencionistas